Abstract
Tight junctions of the pancreatic duct are essential regulators of physiologic secretion of the pancreas and disruption of the pancreatic ductal barrier is known to contribute to the pathogenesis of pancreatitis and progression of pancreatic cancer. Various inflammatory mediators and carcinogens can trigger tight junction disassembly and disruption of the pancreatic barrier, however signaling events that mediates such barrier dysfunctions remain poorly understood. This review focuses on structure and regulation of tight junctions in normal pancreatic epithelial cells and mechanisms of junctional disruption during pancreatic inflammation and cancer. We will pay special attention to a novel model of human telomerase reverse transcriptase-transfected human pancreatic ductal epithelial cells and will describe the roles of major signaling molecules such as protein kinase C and c-Jun N-terminal kinase in formation and disassembly of the pancreatic ductal barrier.
Introduction
Pancreatic duct cells not only deliver the enzymes produced by acinar cells into duodenum but also secrete a HCO3--rich fluid to neutralize gastric acid from the stomach.Citation1 Tight junctions of the pancreatic duct are regulators of physiologic secretion of the pancreas and disruption of the pancreatic ductal barrier. The tight junction, the most apically located of the intercellular junctional complexes, inhibits solute and water flow through the paracellular space (termed the “barrier” function).Citation2,Citation3 It also separates the apical from the basolateral cell surface domains to establish cell polarity (termed the “fence” function).Citation4,Citation5 Tight junctions participate in signal transduction mechanisms that regulate epithelial cell proliferation, gene expression, differentiation and morphogenesis.Citation6 The tight junction is formed by not only the integral membrane proteins claudins, occludin and JAMs, but also many peripheral membrane proteins.Citation7-Citation9 These tight junction proteins are regulated by various cytokines and growth factors via distinct signal transduction pathways.Citation10,Citation11 Normal ductal and acinar structures of the pancreas express claudin-1, -2, -3, -4 and -7, whereas endocrine cells within the islets of Langerhans express claudin-3 and -7.Citation12,Citation13 In the pancreatic duct, freeze-fracture analysis reveals that tight junctions contained a parallel array of three to five continuous sealing strands and the pancreatic enzymes cannot leak out from the lumen into the intercellular spaces.Citation14,Citation15
Pancreatic ductal tight junctions, which is leaky and has the function of selective permeability, may play a role of channels of Na+ and HCO3- via paracellular pathway.Citation16,Citation17 The tight junctions of pancreatic duct epithelial cells and exocrine cells are dynamic structures that can be disrupted by various external stimuli including ductal hypertension.Citation18,Citation19 The disruption of pancreatic duct tight junctions is an early event in different types of pancreatitis.Citation20-Citation25 Although dysfunction of tight junctions in pancreatic duct are observed by various pathological condition, the regulatory mechanisms of tight junctions remain unknown even in normal human pancreatic duct epithelial (HPDE) cells.
On the other hand, in pancreatic cancer, claudin-4 and -18 are highly expressed and are diagnostic or therapeutic targets of monoclonal antibodies against their extracellular loops.Citation26-Citation28 Both the abundance and the subcellular distribution of specific claudin proteins are different between normal and transformed pancreatic epithelia and the changes in paracellular permeability accompany the formation of pancreatic intraepithelial neoplasia (PanIN).Citation29 The claudin family, which consists of at least 27 members, is solely responsible for forming tight junction strands and has four transmembrane domains and two extracellular loops.Citation7,Citation30 The first extracellular loop is the coreceptor of hepatitis C virus and influences the paracellular charge selectivity and the second extracellular loop is the receptor of Clostridium perfringens enterotoxin (CPE).Citation31-Citation33 The 35-kDa polypeptide CPE causes food poisoning in humans, binds to its claudin receptor and then causes changes in membrane permeability via formation of a complex on the plasma membrane followed by the induction of apoptosis.Citation34 In pancreatic cancer, claudin-4 is frequently overexpressed and is a high-affinity receptor of CPE.Citation27,Citation35 It is anticipated that it may be possible to develop a novel tumor-targeted therapy for pancreatic cancer using a claudin-4-targeting molecule.
This review focuses on recent our findings about the relationship between tight junctions and signal transduction pathways in normal human pancreatic duct epithelial cells, using hTERT-transfected human pancreatic epithelial cells ().
Table 1. Changes of tight junction proteins and barrier function in normal human pancreatic duct epithelial cells via PKC and JNK pathways
Tight Junction Molecules of hTERT-HPDE Cells
The introduction of the catalytic subunit of human telomerase, human telomerase reverse transcriptase (hTERT), into human somatic cells such as fibroblasts and retinal pigment epithelial cells typically extends their lifespan without altering their growth requirements, disturbance of the cell-cycle checkpoints, tumorigenicity or chromosomal abnormalities.Citation36-Citation38 We established hTERT-transfected human pancreatic epithelial cells (hTERT-HPDE) with an extended life span.Citation13
The hTERT-HPDE cells are positive for HPDE cell markers such as CK7, CK19 and carbonic anhydrase isozyme 2 (CA-II) and express epithelial tight junction molecules claudin-1, -4, -7 and -18, occludin, tricellulin, marvelD3, JAM-A, ZO-1 and ZO-2.Citation13 The expression patterns of tight junction molecules in the hTERT-HPDE cells are similar to those of pancreatic tissues in vivo.Citation13
Induction of Tight Junction Molecules and the Barrier Function by FBS in hTERT-HPDE Cells
In this culture system, hTERT-HPDE cells in serum-free conditioned medium have growth potential and a long lifespan. Treatment with FBS induces an increase of protein and mRNA of CA-II dependent on the FBS concentration, whereas proteins of CK7 and CK19 are stably expressed independent of the FBS concentration. Claudin-1, -4 and -7, occludin, JAM-A and ZO-1, -2 are induced together with an increase of the barrier function by 10% FBS and the upregulation is inhibited by the pan-PKC inhibitor GF109203X ().Citation13 The tight junction molecules and the barrier function induced by FBS in hTERT-HPDE cells are in part regulated via a PKC pathway.
Barrier Function of hTERT-HPDE Cells and Pancreatic Cancer Cell Lines
In immunocytochemistry, occludin which is a good marker of tight junction position, is localized at the cell membranes of hTERT-HPDE cells with 10% FBS and pancreatic cancer cell lines PANC-1 and BXPC3 (poorly differentiated types), HPAF-II and HPAC (moderately or well-differentiated types), whereas in hTERT-HPDE cells without FBS, it is not detected at the membranes (). When the barrier function was measured by transepithelial electrical resistance (TER) values, the barrier function in hTERT-HPDE cells with 10% FBS was well maintained as well-diffrentiated pancreatic cancer cells HPAF-II and HPAC (). The barrier function in the pancreatic duct may be independent on the localization of occludin.
Figure 1.(A) Immunostaining for occludin and (B) TER values in hTERT-HPDE cells with or without 10% FBS and pancreatic cancer cell lines PANC-1, BXPC-3, HPAF-II and HPAC. Bars: 40 μm. Data represent the mean (n = 6). (C) A line graph for the changes in proteins of claudin-1, -4 and -7 in hTERT-HPDE cells treated with 100 nM TPA. (D) Diagram showing regulation of tight junction molecules via PKC isoforms in hTERT-HPDE cells. CLDN: claudin, OCLN: occludin.
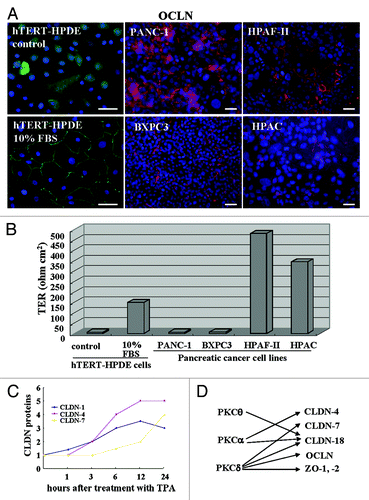
It is thought that normal HPDE cells are sealed well by tight junctions and the tight junctions play a crucial role in the reflux of the exocrine pancreatic juice. The barrier function of well-diffrentiated pancreatic cancer cells is well maintained compared with poorly differentiated pancreatic cancer cells.
Regulation of Tight Junction Molecules by a PKC Activator in hTERT-HPDE Cells
Protein kinase C (PKC) is a family of serine-threonine kinases known to regulate epithelial barrier function via tight junctions.Citation39,Citation40 PKC has been shown to induce both assembly and disassembly of tight junctions depending on the cell type and conditions of activation.Citation40-Citation42 PKC activation can readily disrupt the integrity of pancreatic epithelial tight junctions by causing ROCK-II dependent actomyosin-driven contractility or remodeling of the spectrin-adducin based membrane skeleton.Citation43,Citation44
When hTERT-HPDE cells are treated with the PKC activator 12-O-tetradecanoylphorbol 13-acetate (TPA), claudin-1, -4, -7 and -18, occludin, JAM-A and ZO-1, -2 are increased and the upregulation is inhibited by the pan-PKC inhibitor GF109203X ().Citation13,Citation45
It is thought that claudins are regulated by various factors and that there is differential regulation among claudin family members.Citation9,Citation39,Citation40 When we investigated the time-dependent changes in proteins of claudin-1, -4 and -7 in hTERT-HPDE cells after treatment with TPA, claudin-1 was increased from 1 h, claudin-4 was increased from 3 h, and claudin-7 was increased from 12 h ().
We investigated which PKC isoforms play key roles in the upregulation of tight junction proteins by TPA in hTERT-HPDE cells. The upregulation of tight junction proteins by TPA was inhibited completely by a pan-PKC inhibitor (GF109203X). A PKCθ inhibitor (myristoylated PKCθ pseudosubstrate peptide inhibitor) prevented upregulation of claudin-18 by TPA, a PKCα inhibitor (Gö6976) prevented upregulation of claudin-4 and -18 by TPA, and a PKCδ inhibitor (rottlerin) prevented upregulation of claudin-7, -18, occludin, ZO-1 and ZO-2 by TPA ().
By GeneChip analysis of hTERT-HPDE cells treated with or without TPA, upregulation of one of the ELF (E74-like factor) subfamily of the ETS transcription factors ELF3 was observed.Citation13 It is reported that the expression of claudin-7 in epithelial structures in synovial sarcoma is regulated by ELF3.Citation46 In hTERT-HPDE cells, ELF3 mRNA is increased by TPA and a pan-PKC inhibitor prevents upregulation of ELF3 mRNA by TPA and the upregulation of claudin-7 by TPA is inhibited by knockdown of ELF3 using siRNAs.Citation13 These results suggest that claudin-7 in normal HPDE cells might be regulated via a PKCδ/ELF-3 pathway.
We previously reported that the regulation of tight junctions in normal ductal epithelial cells was closely associated with conventional or novel isoforms of PKC and PKC-induced transcriptional factors.Citation47,Citation48 PKC may be a useful target for pancreatic cancer therapy.Citation49 Further study of the tight junctions of normal HPDE cells via a PKC pathway including isoforms is important for not only physiological regulation of tight junction molecules and the barrier function in normal HPDE cells but also for therapeutic targeting in pancreatic cancer cells.
Regulation of Tight Junction Molecules and the Barrier Function by a PKCα Inhibitor in hTERT-HPDE Cells
At least 12 different isozymes of PKC are known and can be subdivided into three classes (classic or conventional, novel and atypical isozymes) according to their responsiveness to activators.Citation50 In the human intestinal epithelial cell lines HT-29 and Caco-2, stimulation with TLR2 ligands leads to activation of the specific PKC isoforms PKCα and PKCδ and enhances barrier function through translocation of ZO-1 on activation.Citation51 PKCα is considered one of the biomarkers for the diagnosis of cancers, including pancreatic cancer.Citation52,Citation53 We previously reported that the PKCα inhibitor Gö6976 modified claudin-1 and -4 in a well-differentiated pancreatic cancer cell line.Citation54 When hTERT-HPDEs were treated with Gö6976, expression of claudin-1, -4, -7 and occludin, and the barrier function measured as TER values, were significantly increased ().Citation55 The TGF-β-PKC-α-PTEN cascade is a key pathway for pancreatic cancer cells to proliferate and metastasize.Citation56 PKCα inhibitors may be potential therapeutic agents against the malignancy of human pancreatic cancer cells.Citation57
Regulation of Rricellulin by JNK Activators in hTERT-HPDE Cells
c-Jun N-terminal kinase (JNK) activation is essential for disassembly of adherens and tight junctions in human keratinocytes and colonic epithelial cells.Citation58,Citation59 When hTERT-HPDE cells are treated with JNK activators, anisomycin and the proinflammatory cytokines IL-1β, TNFα and IL-1α, only tricellulin expression is significantly increased by all JNK activators, and the upregulation was prevented by the JNK inhibitor SP600125 ().Citation60
Tricellulin was identified as the first marker of the tricellular tight junction, which forms at the meeting points of three cells. It is required for the maintenance of the transepithelial barrier and expressed in both the normal pancreatic duct and pancreatic cancer.Citation61-Citation63 It is one of three members of the tight junction-associated MARVEL protein family (TAMP) and is specific to tricellular tight junctions, whereas the other two members, occludin and marveld3, are localized at bicellular tight junctions.Citation61,Citation64,Citation65 It is possible that the regulation of tricellulin may be more sensitive to the activation of JNK than that of bicellular tight junction proteins in normal HPDE cells.
Regulation of Tight Junction Molecules and the Barrier Function by a JNK Inhibitor in hTERT-HPDE Cells
Recently, it was reported that the JNK inhibitor SP600125 enhanced epithelial barrier function through differential modulation of claudin expression in murine mammary epithelial cells.Citation66 When hTERT-HPDE cells were treated with 10 μM JNK inhibitor SP600125 for 24 h, the cells in phase-contrast images rapidly changed from a cobblestone appearance to a round shape (). This change was similar to that in FBS- or TPA-treated cells.Citation13 In hTERT-HPDE cells after treatment with 10 μM SP600125, claudin-1, -4, occludin and marvelD3 were increased, whereas tricellulin was decreased (). The barrier function measured by the TER values were increased in a time-dependent manner after treatment with 10 μM SP600125 ().
Figure 2.(A) Phase-contrast images of hTERT-HPDE cells treated with 10 μM SP600925. Bar: 40 μm. (B) Western blotting for claudin-1, -4 and -7, OCLN, TRIC, MARVELD3 and E-cadherin in hTERT-HPDE cells treated with 10 μM SP600925. (C) TER values of hTERT-HPDE cells treated with 10 μM SP600925. Data represent the mean ± SD (n = 3). CLDN: claudin, OCLN: occludin, TRIC: tricellulin.
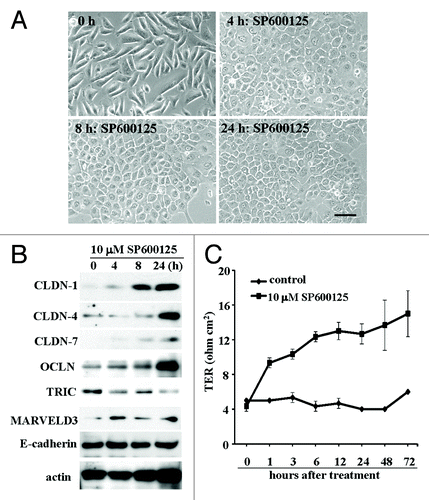
Activation of JNK promotes developments of various tumors.Citation67-Citation69 JNK1-deficient mice exhibits a decrease in carcinogenesis of chemical induced gastric cancer or hepatocellular carcinoma, and JNK2-deficient mice shows reduced skin tumors.Citation70-Citation72 Furthermore, JNK inhibitors decrease growth of human and murine pancreatic cancer in vitro and in vivo.Citation73 JNK may be involved in the regulation of tight junctions, including tricellulin expression and the barrier function in normal pancreatic duct epithelial cells and may be a potential therapeutic target for pancreatic cancer.
Conclusion
Using hTERT-HPDE cells, we indicated that the expression of tight junction molecules and the barrier function in normal HPDE cells were regulated by various factors including PKC and JNK signal pathways (). It is necessary to investigate the detailed regulation of tight junctions in normal HPDE cells via other signal transduction pathways as Hedgehog and Wnt/β-catenin. It is also important that the regulation of MARVEL family members, including tricellulin and marvelD3, be further investigated and compared with that of the claudin family in normal pancreatic duct epithelial cells.Citation74 The profile of tight junctions and the signaling in normal human pancreas may be potential diagnostic or therapeutic targets in inflammation and cancer of pancreas.
Acknowledgments
This work was supported by the Pancreas Research Foundation of Japan and the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare of Japan.
Submitted
01/17/2013
Revised
04/28/2013
Accepted
05/01/2013
Disclosure of Potential Conflicts of Interest
The authors declare no conflicts of interest.
References
- Grapin-Botton A. Ductal cells of the pancreas. Int J Biochem Cell Biol 2005; 37:504 - 10; http://dx.doi.org/10.1016/j.biocel.2004.07.010; PMID: 15618005
- Gumbiner BM. Breaking through the tight junction barrier. J Cell Biol 1993; 123:1631 - 3; http://dx.doi.org/10.1083/jcb.123.6.1631; PMID: 8276885
- Schneeberger EE, Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol 1992; 262:L647 - 61; PMID: 1616050
- van Meer G, Simons K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO J 1986; 5:1455 - 64; PMID: 3743548
- Cereijido M, Valdés J, Shoshani L, Contreras RG. Role of tight junctions in establishing and maintaining cell polarity. Annu Rev Physiol 1998; 60:161 - 77; http://dx.doi.org/10.1146/annurev.physiol.60.1.161; PMID: 9558459
- Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol 2003; 4:225 - 36; http://dx.doi.org/10.1038/nrm1055; PMID: 12612641
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2001; 2:285 - 93; http://dx.doi.org/10.1038/35067088; PMID: 11283726
- Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, et al. Tight junctions and human diseases. Med Electron Microsc 2003; 36:147 - 56; http://dx.doi.org/10.1007/s00795-003-0219-y; PMID: 14505058
- Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 2004; 286:C1213 - 28; http://dx.doi.org/10.1152/ajpcell.00558.2003; PMID: 15151915
- González-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta 2008; 1778:729 - 56; http://dx.doi.org/10.1016/j.bbamem.2007.08.018; PMID: 17950242
- Kojima T, Murata M, Yamamoto T, Lan M, Imamura M, Son S, et al. Tight junction proteins and signal transduction pathways in hepatocytes. Histol Histopathol 2009; 24:1463 - 72; PMID: 19760595
- Borka K, Kaliszky P, Szabó E, Lotz G, Kupcsulik P, Schaff Z, et al. Claudin expression in pancreatic endocrine tumors as compared with ductal adenocarcinomas. Virchows Arch 2007; 450:549 - 57; http://dx.doi.org/10.1007/s00428-007-0406-7; PMID: 17429687
- Yamaguchi H, Kojima T, Ito T, Kimura Y, Imamura M, Son S, et al. Transcriptional control of tight junction proteins via a protein kinase C signal pathway in human telomerase reverse transcriptase-transfected human pancreatic duct epithelial cells. Am J Pathol 2010; 177:698 - 712; http://dx.doi.org/10.2353/ajpath.2010.091226; PMID: 20566751
- Tsukiyama K. Ultrastructure of intercellular junctions in the rat exocrine pancreas stimulated by pancreozymin. Arch Histol Jpn 1979; 42:141 - 52; http://dx.doi.org/10.1679/aohc1950.42.141; PMID: 464749
- Madden ME, Sarras MP Jr.. The pancreatic ductal system of the rat: cell diversity, ultrastructure, and innervation. Pancreas 1989; 4:472 - 85; http://dx.doi.org/10.1097/00006676-198908000-00013; PMID: 2762275
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol 1963; 17:375 - 412; http://dx.doi.org/10.1083/jcb.17.2.375; PMID: 13944428
- Greenwell JR. The selective permeability of the pancreatic duct of the cat to monovalent ions. Pflugers Arch 1977; 367:265 - 70; http://dx.doi.org/10.1007/BF00581364; PMID: 556848
- Akao S, Oya M, Akiyama H, Ishikawa H. The tight junction of pancreatic exocrine cells is a morphometrically dynamic structure altered by intraductal hypertension. J Gastroenterol 2000; 35:758 - 67; http://dx.doi.org/10.1007/s005350070035; PMID: 11063220
- Akao S, Kiumi F. The tight junction of main pancreatic duct epithelial cells is a morphometrically dynamic structure altered by intraductal hypertension. Med Electron Microsc 2002; 35:146 - 52; http://dx.doi.org/10.1007/s007950200018; PMID: 12353135
- Harvey MH, Wedgwood KR, Austin JA, Reber HA. Pancreatic duct pressure, duct permeability and acute pancreatitis. Br J Surg 1989; 76:859 - 62; http://dx.doi.org/10.1002/bjs.1800760832; PMID: 2475200
- Arendt T, Rogos R. Pancreatic exocrine secretion in acute experimental pancreatitis. Gastroenterology 1991; 101:276 - 8; PMID: 2044921
- Fallon MB, Gorelick FS, Anderson JM, Mennone A, Saluja A, Steer ML. Effect of cerulein hyperstimulation on the paracellular barrier of rat exocrine pancreas. Gastroenterology 1995; 108:1863 - 72; http://dx.doi.org/10.1016/0016-5085(95)90151-5; PMID: 7539388
- Schmitt M, Klonowski-Stumpe H, Eckert M, Lüthen R, Häussinger D. Disruption of paracellular sealing is an early event in acute caerulein-pancreatitis. Pancreas 2004; 28:181 - 90; http://dx.doi.org/10.1097/00006676-200403000-00010; PMID: 15028951
- Coskun T, Bozoklu S, Ozenç A, Ozdemir A. Effect of hydrogen peroxide on permeability of the main pancreatic duct and morphology of the pancreas. Am J Surg 1998; 176:53 - 8; http://dx.doi.org/10.1016/S0002-9610(98)00096-8; PMID: 9683134
- Rotoli BM, Orlandini G, Guizzardi S, Uggeri J, Dall’Asta V, Gazzola GC, et al. Ethanol increases the paracellular permeability of monolayers of CAPAN-1 pancreatic duct cells. J Mol Histol 2004; 35:355 - 62; http://dx.doi.org/10.1023/B:HIJO.0000039838.56131.02; PMID: 15503809
- Michl P, Barth C, Buchholz M, Lerch MM, Rolke M, Holzmann KH, et al. Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res 2003; 63:6265 - 71; PMID: 14559813
- Michl P, Buchholz M, Rolke M, Kunsch S, Löhr M, McClane B, et al. Claudin-4: a new target for pancreatic cancer treatment using Clostridium perfringens enterotoxin. Gastroenterology 2001; 121:678 - 84; http://dx.doi.org/10.1053/gast.2001.27124; PMID: 11522752
- Karanjawala ZE, Illei PB, Ashfaq R, Infante JR, Murphy K, Pandey A, et al. New markers of pancreatic cancer identified through differential gene expression analyses: claudin 18 and annexin A8. Am J Surg Pathol 2008; 32:188 - 96; http://dx.doi.org/10.1097/PAS.0b013e31815701f3; PMID: 18223320
- Westmoreland JJ, Drosos Y, Kelly J, Ye J, Means AL, Washington MK, et al. Dynamic distribution of claudin proteins in pancreatic epithelia undergoing morphogenesis or neoplastic transformation. Dev Dyn 2012; 241:583 - 94; http://dx.doi.org/10.1002/dvdy.23740; PMID: 22275141
- Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, et al. Predicted expansion of the claudin multigene family. FEBS Lett 2011; 585:606 - 12; http://dx.doi.org/10.1016/j.febslet.2011.01.028; PMID: 21276448
- Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wölk B, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 2007; 446:801 - 5; http://dx.doi.org/10.1038/nature05654; PMID: 17325668
- Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol 2006; 68:403 - 29; http://dx.doi.org/10.1146/annurev.physiol.68.040104.131404; PMID: 16460278
- Fujita K, Katahira J, Horiguchi Y, Sonoda N, Furuse M, Tsukita S. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett 2000; 476:258 - 61; http://dx.doi.org/10.1016/S0014-5793(00)01744-0; PMID: 10913624
- McClane BA, Chakrabarti G. New insights into the cytotoxic mechanisms of Clostridium perfringens enterotoxin. Anaerobe 2004; 10:107 - 14; http://dx.doi.org/10.1016/j.anaerobe.2003.11.004; PMID: 16701507
- Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J Biol Chem 1997; 272:26652 - 8; http://dx.doi.org/10.1074/jbc.272.42.26652; PMID: 9334247
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. Extension of life-span by introduction of telomerase into normal human cells. Science 1998; 279:349 - 52; http://dx.doi.org/10.1126/science.279.5349.349; PMID: 9454332
- Vaziri H, Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol 1998; 8:279 - 82; http://dx.doi.org/10.1016/S0960-9822(98)70109-5; PMID: 9501072
- Kurose M, Kojima T, Koizumi J, Kamekura R, Ninomiya T, Murata M, et al. Induction of claudins in passaged hTERT-transfected human nasal epithelial cells with an extended life span. Cell Tissue Res 2007; 330:63 - 74; http://dx.doi.org/10.1007/s00441-007-0453-z; PMID: 17701057
- Balda MS, Gonzalez-Mariscal L, Matter K, Cereijido M, Anderson JM. Assembly of the tight junction: the role of diacylglycerol. J Cell Biol 1993; 123:293 - 302; http://dx.doi.org/10.1083/jcb.123.2.293; PMID: 8408213
- Andreeva AY, Piontek J, Blasig IE, Utepbergenov DI. Assembly of tight junction is regulated by the antagonism of conventional and novel protein kinase C isoforms. Int J Biochem Cell Biol 2006; 38:222 - 33; PMID: 16257565
- Ellis B, Schneeberger EE, Rabito CA. Cellular variability in the development of tight junctions after activation of protein kinase C. Am J Physiol 1992; 263:F293 - 300; PMID: 1324609
- Sjö A, Magnusson KE, Peterson KH. Distinct effects of protein kinase C on the barrier function at different developmental stages. Biosci Rep 2003; 23:87 - 102; http://dx.doi.org/10.1023/A:1025524323842; PMID: 14570379
- Ivanov AI, Samarin SN, Bachar M, Parkos CA, Nusrat A. Protein kinase C activation disrupts epithelial apical junctions via ROCK-II dependent stimulation of actomyosin contractility. BMC Cell Biol 2009; 10:36; http://dx.doi.org/10.1186/1471-2121-10-36; PMID: 19422706
- Naydenov NG, Ivanov AI. Adducins regulate remodeling of apical junctions in human epithelial cells. Mol Biol Cell 2010; 21:3506 - 17; http://dx.doi.org/10.1091/mbc.E10-03-0259; PMID: 20810786
- Ito T, Kojima T, Yamaguchi H, Kyuno D, Kimura Y, Imamura M, et al. Transcriptional regulation of claudin-18 via specific protein kinase C signaling pathways and modification of DNA methylation in human pancreatic cancer cells. J Cell Biochem 2011; 112:1761 - 72; http://dx.doi.org/10.1002/jcb.23095; PMID: 21381080
- Kohno Y, Okamoto T, Ishibe T, Nagayama S, Shima Y, Nishijo K, et al. Expression of claudin7 is tightly associated with epithelial structures in synovial sarcomas and regulated by an Ets family transcription factor, ELF3. J Biol Chem 2006; 281:38941 - 50; http://dx.doi.org/10.1074/jbc.M608389200; PMID: 17060315
- Koizumi J, Kojima T, Ogasawara N, Kamekura R, Kurose M, Go M, et al. Protein kinase C enhances tight junction barrier function of human nasal epithelial cells in primary culture by transcriptional regulation. Mol Pharmacol 2008; 74:432 - 42; http://dx.doi.org/10.1124/mol.107.043711; PMID: 18477669
- Ogasawara N, Kojima T, Go M, Ohkuni T, Koizumi J, Kamekura R, et al. PPARgamma agonists upregulate the barrier function of tight junctions via a PKC pathway in human nasal epithelial cells. Pharmacol Res 2010; 61:489 - 98; http://dx.doi.org/10.1016/j.phrs.2010.03.002; PMID: 20227502
- Ali AS, Ali S, El-Rayes BF, Philip PA, Sarkar FH. Exploitation of protein kinase C: a useful target for cancer therapy. Cancer Treat Rev 2009; 35:1 - 8; http://dx.doi.org/10.1016/j.ctrv.2008.07.006; PMID: 18778896
- Newton AC. Regulation of protein kinase C. Curr Opin Cell Biol 1997; 9:161 - 7; http://dx.doi.org/10.1016/S0955-0674(97)80058-0; PMID: 9069266
- Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 2004; 127:224 - 38; http://dx.doi.org/10.1053/j.gastro.2004.04.015; PMID: 15236188
- Kang JH, Asai D, Toita R, Kitazaki H, Katayama Y. Plasma protein kinase C (PKC)alpha as a biomarker for the diagnosis of cancers. Carcinogenesis 2009; 30:1927 - 31; http://dx.doi.org/10.1093/carcin/bgp210; PMID: 19710177
- Denham DW, Franz MG, Denham W, Zervos EE, Gower WR Jr., Rosemurgy AS, et al. Directed antisense therapy confirms the role of protein kinase C-alpha in the tumorigenicity of pancreatic cancer. Surgery 1998; 124:218 - 23, discussion 223-4; http://dx.doi.org/10.1016/S0039-6060(98)70123-0; PMID: 9706141
- Kyuno D, Kojima T, Ito T, Yamaguchi H, Tsujiwaki M, Takasawa A, et al. Protein kinase Cα inhibitor enhances the sensitivity of human pancreatic cancer HPAC cells to Clostridium perfringens enterotoxin via claudin-4. Cell Tissue Res 2011; 346:369 - 81; http://dx.doi.org/10.1007/s00441-011-1287-2; PMID: 22160590
- Kyuno D, Kojima T, Yamaguchi H, Ito T, Kimura Y, Imamura M, et al. Protein kinase Cα inhibitor protects against downregulation of claudin-1 during epithelial-mesenchymal transition of pancreatic cancer. Carcinogenesis 2013; http://dx.doi.org/10.1093/carcin/bgt057; PMID: 23389293
- Chow JY, Dong H, Quach KT, Van Nguyen PN, Chen K, Carethers JM. TGF-beta mediates PTEN suppression and cell motility through calcium-dependent PKC-alpha activation in pancreatic cancer cells. Am J Physiol Gastrointest Liver Physiol 2008; 294:G899 - 905; http://dx.doi.org/10.1152/ajpgi.00411.2007; PMID: 18239055
- Konopatskaya O, Poole AW. Protein kinase Calpha: disease regulator and therapeutic target. Trends Pharmacol Sci 2010; 31:8 - 14; http://dx.doi.org/10.1016/j.tips.2009.10.006; PMID: 19969380
- Naydenov NG, Hopkins AM, Ivanov AI. c-Jun N-terminal kinase mediates disassembly of apical junctions in model intestinal epithelia. Cell Cycle 2009; 8:2110 - 21; http://dx.doi.org/10.4161/cc.8.13.8928; PMID: 19502798
- Lee MH, Koria P, Qu J, Andreadis ST. JNK phosphorylates beta-catenin and regulates adherens junctions. FASEB J 2009; 23:3874 - 83; http://dx.doi.org/10.1096/fj.08-117804; PMID: 19667122
- Kojima T, Fuchimoto J, Yamaguchi H, Ito T, Takasawa A, Ninomiya T, et al. c-Jun N-terminal kinase is largely involved in the regulation of tricellular tight junctions via tricellulin in human pancreatic duct epithelial cells. J Cell Physiol 2010; 225:720 - 33; http://dx.doi.org/10.1002/jcp.22273; PMID: 20533305
- Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 2005; 171:939 - 45; http://dx.doi.org/10.1083/jcb.200510043; PMID: 16365161
- Krug SM, Amasheh S, Richter JF, Milatz S, Günzel D, Westphal JK, et al. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell 2009; 20:3713 - 24; http://dx.doi.org/10.1091/mbc.E09-01-0080; PMID: 19535456
- Korompay A, Borka K, Lotz G, Somorácz A, Törzsök P, Erdélyi-Belle B, et al. Tricellulin expression in normal and neoplastic human pancreas. Histopathology 2012; 60:6B E76 - 86; http://dx.doi.org/10.1111/j.1365-2559.2012.04189.x; PMID: 22394074
- Steed E, Rodrigues NT, Balda MS, Matter K. Identification of MarvelD3 as a tight junction-associated transmembrane protein of the occludin family. BMC Cell Biol 2009; 10:95; http://dx.doi.org/10.1186/1471-2121-10-95; PMID: 20028514
- Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, et al. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell 2010; 21:1200 - 13; http://dx.doi.org/10.1091/mbc.E09-08-0734; PMID: 20164257
- Carrozzino F, Pugnale P, Féraille E, Montesano R. Inhibition of basal p38 or JNK activity enhances epithelial barrier function through differential modulation of claudin expression. Am J Physiol Cell Physiol 2009; 297:C775 - 87; http://dx.doi.org/10.1152/ajpcell.00084.2009; PMID: 19605737
- Sancho R, Nateri AS, de Vinuesa AG, Aguilera C, Nye E, Spencer-Dene B, et al. JNK signalling modulates intestinal homeostasis and tumourigenesis in mice. EMBO J 2009; 28:1843 - 54; http://dx.doi.org/10.1038/emboj.2009.153; PMID: 19521338
- Cui J, Han SY, Wang C, Su W, Harshyne L, Holgado-Madruga M, et al. c-Jun NH(2)-terminal kinase 2alpha2 promotes the tumorigenicity of human glioblastoma cells. Cancer Res 2006; 66:10024 - 31; http://dx.doi.org/10.1158/0008-5472.CAN-06-0136; PMID: 17047065
- Yang YM, Bost F, Charbono W, Dean N, McKay R, Rhim JS, et al. C-Jun NH(2)-terminal kinase mediates proliferation and tumor growth of human prostate carcinoma. Clin Cancer Res 2003; 9:391 - 401; PMID: 12538493
- Shibata W, Maeda S, Hikiba Y, Yanai A, Sakamoto K, Nakagawa H, et al. c-Jun NH2-terminal kinase 1 is a critical regulator for the development of gastric cancer in mice. Cancer Res 2008; 68:5031 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-07-6332; PMID: 18593901
- Hui L, Zatloukal K, Scheuch H, Stepniak E, Wagner EF. Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation. J Clin Invest 2008; 118:3943 - 53; http://dx.doi.org/10.1172/JCI37156; PMID: 19033664
- Chen N, Nomura M, She QB, Ma WY, Bode AM, Wang L, et al. Suppression of skin tumorigenesis in c-Jun NH(2)-terminal kinase-2-deficient mice. Cancer Res 2001; 61:3908 - 12; PMID: 11358804
- Takahashi R, Hirata Y, Sakitani K, Nakata W, Kinoshita H, Hayakawa Y, et al. Therapeutic effect of c-Jun N-terminal kinase inhibition on pancreatic cancer. Cancer Sci 2013; 104:337 - 44; http://dx.doi.org/10.1111/cas.12080; PMID: 23237571
- Kojima T, Takasawa A, Kyuno D, Ito T, Yamaguchi H, Hirata K, et al. Downregulation of tight junction-associated MARVEL protein marvelD3 during epithelial-mesenchymal transition in human pancreatic cancer cells. Exp Cell Res 2011; 317:2288 - 98; http://dx.doi.org/10.1016/j.yexcr.2011.06.020; PMID: 21763689