Abstract
In eukaryotes, mRNA translation is dependent on the cap-binding protein eIF4E. Through its simultaneous interaction with the mRNA cap structure and with the ribosome-associated eIF4G adaptor protein, eIF4E physically posits the ribosome at the 5′ extremity of capped mRNA. eIF4E activity is regulated by phosphorylation on a unique site by the eIF4G-associated kinase MNK. eIF4E assembly with the eIF4G-MNK sub-complex can be however antagonized by the hypophosphorylated forms of eIF4E-binding protein (4E-BP). We show here that eIF4E phosphorylation is dramatically affected by disruption of eIF4E-eIF4G interaction, independently of changes in MNK expression. eIF4E phosphorylation is actually strongly downregulated upon eIF4G shutdown or upon sequestration by hypophosphorylated 4E-BP, consequent to mTOR inhibition. Downregulation of 4E-BP renders eIF4E phosphorylation insensitive to mTOR inhibition. These data highlight the important role of 4E-BP in regulating eIF4E phosphorylation independently of changes in MNK expression.
Introduction
In eukaryotes, capped mRNAs can interact with the cap-binding protein eIF4E (eukaryotic (translation) initiation factor 4E).Citation1 Different functions have been assigned to eIF4E including a role in mRNA transport, degradation or translation into proteins.Citation2 The translational function of eIF4E is the target of different mechanisms of regulation. eIF4E indeed interacts with the translation stimulatory proteins eIF4GI or eIF4GII (hereafter indifferently referred as eIF4G), or with different inhibitors of translation such as the eIF4E-binding protein 1 (4E-BP1) or 4E-BP2 (hereafter indifferently referred as 4E-BP).Citation3 In addition to these regulatory interactions, eIF4E activity is also controlled by phosphorylation on a unique site (S209 in the human sequence). Through its role in the stimulation of protein synthesis, eIF4E has been implicated in tumorigenesis,Citation4 and S209 phosphorylation has been shown to be required for eIF4E’s oncogenic potential.Citation5,Citation6 The S209 residue is phosphorylated mainly by the MAPK-interacting protein kinase 1 (MNK1) or MNK2 (hereafter indifferently referred as MNK).Citation7 Consistently, the deficiency of both MNK1 and MNK2 kinases delays tumor progression.Citation8 Interestingly, S209 phosphorylation appears facilitated when eIF4E is bound to eIF4G. Indeed, the N-terminal end of MNK interacts with the C-terminal extremity of eIF4G.Citation9 Therefore, the simultaneous binding of eIF4E and MNK to their common partner eIF4G facilitates eIF4E phosphorylation by MNK.Citation10 These characteristics suggest that the control of eIF4E phosphorylation may not strictly depends on changes in MNK expression and/or activity, but also on changes in the interaction between eIF4E and the eIF4G-MNK sub-complex, an interaction itself modulated by 4E-BP.
4E-BP inhibits translation initiation by competing with eIF4G for a common binding site on eIF4E.Citation11 Consistently, overexpression of 4E-BP prevents eIF4E-induced tumorigenesis.Citation12,Citation13 The interaction of 4E-BP with eIF4E is however precluded when 4E-BP is phosphorylated by mTOR,Citation14 a kinase which lies downstream of the growth-factor-dependent PI3K/AKT pathway. mTOR is also a sensor of amino-acid availability. Upon amino-acid starvation (or under treatment with mTOR inhibitors), 4E-BP is no longer phosphorylated, sequesters eIF4E and inhibits protein synthesis. It can be therefore anticipated that eIF4E sequestration by 4E-BP and consequent eviction of the eIF4G-MNK sub-complex negatively impinges upon eIF4E phosphorylation, independently of changes in MNK status. This is supported by the findings that tumorigenesis induced after homozygous deletion of Pten, a tumor suppressor gene that prevents 4E-BP phosphorylation through inhibition of the PI3K/AKT/mTOR pathway, appears strongly dependent on eIF4E phosphorylation.Citation8 To address this question directly, we have here compared the capacity of eIF4E to be (de)phosphorylated in different cell types upon manipulation of eIF4E-eIF4G-MNK complex assembly by targeting 4E-BP (mTOR inhibition, shRNAs or knockout mice), eIF4G (shRNAs) or MNK (shRNAs or pharmacological inhibitors).
Results and Discussion
One observation that led us to investigate the potential role of 4E-BP in eIF4E phosphorylation is that, in addition to hypophosphorylation of 4E-BP, mTOR inactivation by its specific inhibitor PP242 impairs eIF4E phosphorylation (, lanes 1 and 2; and Furic et al.Citation6). Yet, eIF4E is not a substrate of mTOR. However, the functional consequence of 4E-BP hypophosphorylation upon PP242-mediated mTOR inactivation is the binding of eIF4E to 4E-BP instead of eIF4G, as visualized by a cap-column assay (lanes 3–5) or by eIF4G immunoprecipitation (lanes 6 and 7). Because the eIF4E kinase MNK is associated with eIF4G, MNK is also separated from its substrate eIF4E.Citation10 We therefore hypothesized that eviction of the eIF4G-MNK sub-complex due to eIF4E sequestration by the hypophosphorylated forms of 4E-BP was responsible for the inhibition of eIF4E phosphorylation upon mTOR inactivation. To explore this hypothesis, we first looked at eIF4E phosphorylation status in proliferating HEK cells (i.e., in the presence of 10% fetal calf serum or FCS) where 4E-BP1 and/or 4E-BP2 expression has been downregulated by specific shRNAs. No significant changes in the level of eIF4E phosphorylation were detected upon downregulation of either 4E-BP1 or 4E-BP2 or both ( left). In these conditions however eIF4E phosphorylation appeared not affected probably because in the presence of FCS, 4E-BP1 and 4E-BP2 are hyperphosphorylated and are therefore not expected to sequester eIF4E. Consistently, when the experiment was reproduced in the presence of the mTOR inhibitor PP242, the impairment of eIF4E phosphorylation following mTOR inhibition was much less marked when 4E-BP1 or 4E-BP2 or both proteins were downregulated ( right). The use of specific shRNAs in HEK cells did not permit a full extinction of 4E-BP1 or 4E-BP2 expression. We therefore checked the status of eIF4E phosphorylation in proliferating mouse embryonic fibroblasts (MEFs) where the two 4e-bp1 and 2 loci are ablated (hereafter named 4E-BP DKO MEFs). The levels of eIF4E phosphorylation appeared to be similar in 4E-BP DKO MEFs and in wild-type (WT) MEFs (, lanes 1 and 3). However, as described above for HEK cells, when the phosphorylation of 4E-BP was blocked by PP242-mediated inhibition of mTOR, eIF4E phosphorylation remained unchanged in 4E-BP DKO MEFs but was dramatically decreased in WT MEFs (, lanes 2 and 4). These data indicated that impairment of eIF4E phosphorylation upon mTOR inactivation (by PP242) occurs solely when cells express 4E-BP1 and/or 4E-BP2, suggesting a crucial role of eIF4E/eIF4G interaction in the regulation of eIF4E phosphorylation. To directly test this hypothesis, we then monitored the effect of eIF4GI and/or eIF4GII extinction on eIF4E phosphorylation. The use of a combination of doxycycline-inducible shRNAs targeting eIF4GI and/or eIF4GII revealed different contributions of eIF4GI and eIF4GII in the extent of eIF4E phosphorylation. Extinction of eIF4GI strongly decreased eIF4E phosphorylation (, lanes 1 and 2) whereas extinction of eIF4GII had no effect (lanes 1 and 3) presumably because eIF4GII amount in mammalian cells is much lower than that of eIF4GI.Citation15 A similar effect on eIF4E phosphorylation was obtained upon knockdown of both eIF4GI and eIF4GII compared with eIF4GI silencing alone (, lanes 2 and 4).
Figure 1. eIF4E and 4E-BP1 are dephosphorylated upon mTOR inhibition by PP242. HeLa cells were treated with 2.5 μM of PP242 for 1 h or left untreated. Whole cell lysates were incubated with m7GTP-beads (cap column), beads alone (beads) or subjected to immunoprecipitation with eIF4GI antibody (IP eIF4GI). Bound proteins and whole cell lysates (input) were separated by SDS-PAGE and analyzed by western blotting with the indicated antibodies. Data are representative of at least three independent experiments.
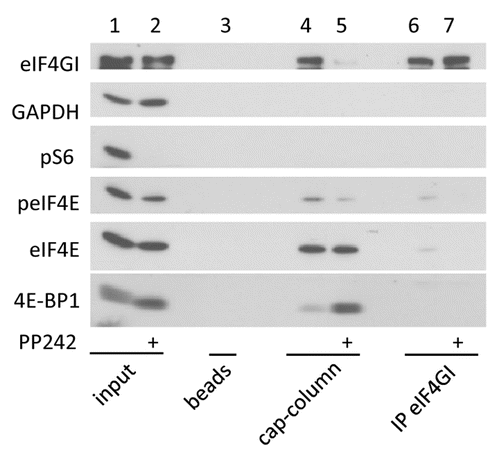
Figure 2. eIF4E phosphorylation is favored by 4E-BP downregulation but hindered by eIF4G silencing. (A) HEK cells expressing shRNA against 4E-BP1 and/or 4E-BP2 or Scramble shRNA were treated with 2.5 μM of PP242 for 1 h or left untreated in the presence of 10% FCS. Black arrowhead indicates 4E-BP2 specific signal. Asterisk shows non-specific signal from dephosphorylated 4E-BP1. (B) 4E-BP1/4E-BP2 double knockout (4E-BP DKO) and wild type (WT) mouse embryonic fibroblasts were treated as in (A). (C) MDA-MB231 cells expressing inducible sh-miRNA against eIF4GI and/or eIF4GII or control vector were grown with 5 μg/ml doxycycline for 3 d. Cell lysates were analyzed by western blotting using the indicated antibodies. Data are representative of at least three independent experiments.
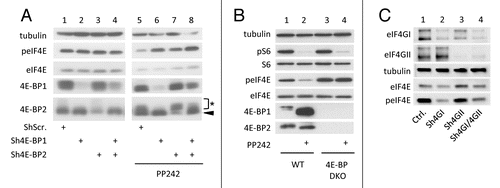
In the experiments described above, the inhibitory role of 4E-BP or the stimulatory role of eIF4G on eIF4E phosphorylation was observed using artificial manipulations of 4E-BP or eIF4G cellular contents (shRNAs or KO mice). We have therefore analyzed the regulation of eIF4E phosphorylation in transformed cells which naturally express different levels of 4E-BP. MiaPaca-2 and Panc-1 pancreatic cancer cells were chosen because they do not express 4E-BP2, as compared with HEK cells. Panc-1 cells express much less 4E-BP1 than MiaPaca-2 and both contain comparable amounts of eIF4E, eIF4GI and MNK1, while MNK2 is not detectable (). Consistently with the observations made in , the extent of eIF4E phosphorylation (lower in MiaPaca-2 than in Panc-1 cells) was inversely correlated with the cellular content of 4E-BP1 (higher in MiaPaca-2 than in Panc-1 cells) (). Also, inhibition of eIF4E phosphorylation by PP242-mediated inactivation of mTOR occurred in MiaPaca-2 cells expressing 4E-BP1 (, lanes 1 and 2) but not in Panc-1 cells poorly expressing 4E-BP1 (, lanes 3 and 4). Thus, alterations in eIF4E Ser209 consequent to the sole destruction of eIF4E-EIF4G interaction appeared dependent on eIF4E accessibility to its kinase MNK. To further explore this hypothesis, the relative importance of 4E-BPs to that of MNK activity or expression level in eIF4E phosphorylation was examined by manipulating MNK activity or expression using MNK pharmacological inhibitors or specific shRNA.
Figure 3. low amount of 4E-BP favors eIF4E phosphorylation. (A) MiaPaca-2, Panc-1 and HEK cells lysates were analyzed by western blotting. (B) MiaPaca-2, Panc-1 were treated with 2.5 μM of PP242 for 1 h. Cell lysates were analyzed by western blotting using the indicated antibodies. Data are representative of at least three independent experiments.
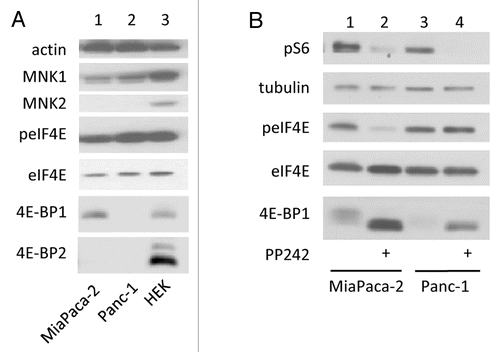
At least two potent inhibitors of MNK activity have been described: CGP57380 and cercosporamide (Reviewed in Hou et al.Citation16). In our hands, we found that CGP57380 used at 20 µM was sufficient to completely abrogate eIF4E phosphorylation independently of changes in 4E-BP1 level or phosphorylation (, lanes 1–3), and was more potent than cercosporamide used at similar concentrations (lanes 4–5). Furthermore, treatment with cercosporamide increased 4E-BP1 phosphorylation in HEK cells, a side effect which could favor eIF4E accessibility to eIF4G thus rendering subsequent conclusions difficult. Overall, the data obtained in HEK cells where 4E-BP1 and/or 4E-BP2 expression has been diminished by specific shRNAs (), those obtained in WT or 4E-BP DKO MEFs (, lanes 1, 3, 4 and 6) or those obtained in pancreatic cancer cell lines (MiaPaca-2 and Panc-1; , lanes 1, 2, 5, 6) revealed that eIF4E phosphorylation is actually sensitive to CGP57380, thus indicating that MNK1 and/or MNK2 remain the major kinases of eIF4E in these conditions. However, these data also revealed that sensitivity of eIF4E phosphorylation to the MNK inhibitor CGP57380 is dependent on 4E-BP1 and/or 4E-BP2 levels. Indeed, eIF4E dephosphorylation upon inhibition of MNK activity was less marked in HEK cells expressing lower levels of 4E-BP1 and 4E-BP2 (), in 4E-BP DKO MEFs (, lanes 3 and 6) and in Panc-1 cells expressing low levels of 4E-BP1 (and devoid of 4E-BP2; , lanes 2 and 6). This observation further supports the notion that eIF4E is efficiently phosphorylated when fully accessible and not sequestered by 4E-BP. We also revealed that, in the presence of 4E-BP1 and 4E-BP2 (WT MEFs), PP242-mediated inhibition of mTOR activity was nearly as efficient as CGP57380-mediated inhibition of MNK activity in preventing eIF4E phosphorylation (, lanes 2 and 3). This suggests that MNK access to eIF4E limits the latter phosphorylation. Nonetheless, limited MNK expression could also participate to these observations. To address this question, we altered MNK expression using shRNA. In MNK1 and MNK2 double knockout mice, eIF4E is not phosphorylated at all.Citation17 We therefore anticipated that MNK1 extinction using specific shRNAs in pancreatic cancer cells (combined with the absence of MNK2 in these cells) should be sufficient to impair eIF4E phosphorylation. Surprisingly however, moderate changes in the extent of eIF4E phosphorylation could be detected upon severe downregulation of MNK1 expression in both MiaPaca-2 and Panc-1 cells (, lanes 1, 3, 5 and 7). In contrast, CGP57380-mediated inhibition of MNK1 strongly inhibited eIF4E phosphorylation (, lanes 2, 4, 6 and 8) demonstrating that MNK1 is present in large excess and is not limiting for eIF4E phosphorylation.
Figure 4. 4E-BPs downregulation does not confer resistance to the MNK inhibitor CGP57380. (A) HEK cells were treated for 1 h with 10 or 20 µM of CGP57380 (CGP.) or cercosporamide (Cerco.) or left untreated. (B) HEK cells expressing shRNA against 4E-BP1 and/or 4E-BP2 or Scramble shRNA were treated with 20 μM of CGP57380 (CGP.) for 1 h or left untreated. (C) 4E-BP DKO and WT MEF were treated with 2.5 μM of PP242 or with 20 µM of CGP57380 for 1 h or left untreated. (D) MiaPaca-2 and Panc-1 cells expressing shRNA against the 3′UTR of MNK1 mRNA or Scramble shRNA were treated with 20µM of CGP for 1 h prior harvesting. Cell lysates were analyzed by western blotting using the indicated antibodies. Data are representative of at least three independent experiments.
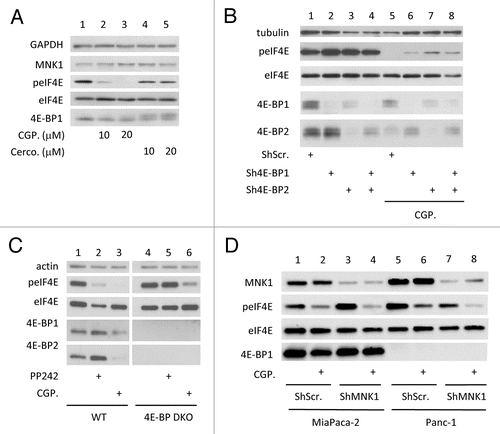
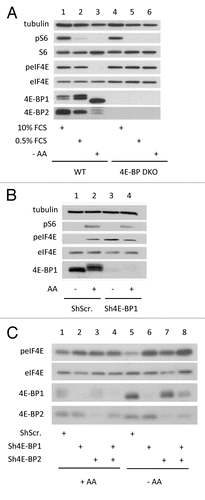
Finally, we have verified whether the observations made above could also be visualized in a more physiological situation. The data showing that pharmacological inhibition of mTOR (PP242-treatment of MEFs) indirectly impinges upon eIF4E phosphorylation through its sequestration by hypophosphorylated 4E-BPs were reproduced when cells were exposed to low concentrations of serum (FCS) or deprivation in amino-acids (AA). eIF4E phosphorylation was indeed much more inhibited under low concentrations of FCS or AA deprivation when MEFs express 4E-BP1 and 4E-BP2 (). Similarly, we found that in MiaPaca-2 cells expressing ShRNA to 4E-BP1 () or in HEK cells where 4E-BP1 and/or 4E-BP2 expression has been diminished by specific shRNAs (), eIF4E phosphorylation was resistant to amino-acid starvation. Thus, combined with the observations that eIF4E phosphorylation is higher in pancreatic cancer cells poorly expressing 4E-BP1 and 4E-BP2, these important results indicate that in the absence of 4E-BPs, eIF4E phosphorylation becomes constitutive and no longer modulated by serum or amino-acid deprivation.
Figure 5. 4E-BPs downregulation renders eIF4E phosphorylation insensitive to serum or amino acids starvation. (A) 4E-BP DKO and WT MEF were incubated overnight in DMEM containing low serum concentration (0.5% FCS) or incubated for 1 h in amino acid-free HBSS medium or left untreated (10% FCS). (B) MiaPaca-2 cells expressing shRNA to 4E- BP1 or Scramble shRNA were incubated for 2 h in amino acid-free HBSS medium or supplemented with 10% FCS and amino acids. Cell lysates were analyzed by western blotting using the indicated antibodies. (C) HEK cells expressing shRNA against 4E-BP1 and/or 4E-BP2 or Scramble shRNA were incubated for 1 h in amino acid-free HBSS medium (-AA) or left untreated in the presence of 10% FCS (+AA). Data are representative of at least three independent experiments.
In summary, these results highlight the important role played by 4E-BP interaction with eIF4E in the control of eIF4E phosphorylation. They also reveal that the intracellular amounts of MNK1 and MNK2 are not limiting (at least in HEK, MEF and pancreatic cancer cells), but rather that MNK accessibility to eIF4E, through concomitant binding of MNK and eIF4E to eIF4G, plays a prominent role in the regulation of eIF4E phosphorylation. Conversely, restricting access to eIF4E via 4E-BP appears to limit the phosphorylation of the cap-binding protein. As eIF4E phosphorylation promotes tumorigenesis, this finding also emphasizes the anti-oncogenic potential of 4E-BP.Citation3
Materials and Methods
Reagents
Antibodies used are as follows: Anti-eIF4GI, anti-eIF4GII were obtained from Dr. N. Sonenberg (McGill University, Montreal, Canada). Anti-eIF4E, 4E-BP1 (9452), 4E-BP2, MNK1 and phospho-ser 240–244 RPS6 (pS6) were from Cell Signaling Technology. Anti-RPS6 (S6), GAPDH and MNK2 were from Santa Cruz. Anti-phospho-ser209-eIF4E (peIF4E) was from Epitomics. Anti-β-tubulin and β-actin were from Sigma-Aldrich. Protein G Sepharose for fast flow and 7-methyl GTP Sepharose 4B were from GE healthcare. Doxycycline, puromycin, gentamicin, CGP57380 and PP242 were from Sigma-Aldrich and Cercosporamide from Santa-Cruz.
Cell culture
HeLa S3 and HEK 293 cells were obtained from the American Tissue Culture Collection and maintained in Dulbecco’s minimum essential medium (DMEM, Lonza) supplemented with 10% fetal bovine serum (FBS, Lonza) and 5 U/ml penicillin-streptomycin (Lonza) in 5% CO2.
Panc-1, MiaPaca-2 pancreatic cancer cell lines and MiaPaca-2 cells expressing shRNA to 4E-BP1 and Non-Target shRNA (scramble) were cultured as previously described.Citation18 shRNA vector accession numbers are: 4E-BP1 TRCN0000040203, 4E-BP2 TRCN0000117814, MNK1 TRCN0000195343 and Non-Target shRNA Control (Scramble) SHC002. MDA-MB231 cells expressing inducible sh-miRNA were obtained by lentiviral transduction of pTRIPZ vectors (Open Biosystems). Targeting sequences were as follows sh4G1mir1: GTAGTGTGATGTGTCTGAACT, sh4G2mir1: AAGTTTCACGTCTTCGCCAAT, Non-Silencing (Ctrl.): AATTCTCCGAACGTGTCACGT. 4E-BP1/4E-BP2 double knockout (DKO) and wild type (WT) Mouse embryonic fibroblasts (MEFs) were obtained from Dr. N. Sonenberg (McGill University, Montreal, Canada). Cells were amino acid-starved for 1 h using HBSS with calcium and magnesium supplemented with 4.5 g/l glucose (Lonza).
Cap-column and immunoprecipitation
Hela S3 cells were seeded in 150 mm plates and treated with a vehicle (DMSO) or PP242 (2.5 µM) for 1 h. Cells were then washed with cold PBS, collected, and lysed in buffer A containing 40 mM HEPES-KOH (pH 7.5), 120 mM NaCl, 1 mM EDTA, 0.1 mM GDP and 10 mM pyrophosphate, 50 mM NaF, and 0.3% CHAPS supplemented with protease and phosphatase inhibitors (as described inCitation19). Cell extracts were transferred onto 7-methyl GTP Sepharose 4B or protein G-Sepharose beads supplemented or not with anti-eIF4GI antibody, and incubated for 2 h at 4°C. Beads were washed four times in buffer A. Bound proteins were eluted with Laemmli buffer and processed for western blotting.
SDS-PAGE and western blotting
Cells were harvested on ice, washed twice with cold PBS, and lysed in 50 mM TRIS-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% NP-40 supplemented with protease and phosphatase inhibitors. Protein concentration was measured using Protein Assay reagent (Bio-Rad), and equal amounts of proteins were subjected to SDS-PAGE and transferred onto nitrocellulose membrane (BioTraceNT; Pall Corp). Membranes were washed in Tris Buffer Saline supplemented with 0.1% Tween 20 (TBS-T) then saturated in TBS-T with 5% non-fat dry milk, incubated overnight with primary antibodies in TBS-T with 5% BSA, washed and revealed according to Cell Signaling Technology protocol.
| Abbreviations: | ||
| MNK | = | MAPK-interacting protein kinase |
| eIF4E | = | eukaryotic translation initiation factor 4E |
| 4E-BP | = | eIF4E-binding protein |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from INSERM-Université Paul Sabatier and from La LIGUE to S. Pyronnet (“Comités de Hautes-Pyrénées et de Lot-et-Garonne”). Y. Martineau was a recipient of Fondation de France post-doctoral fellowships. D. Müller and C. Lasfargues were recipients of doctoral fellowships from La Ligue Nationale Contre le Cancer. A. Alard was the recipient of a fellowship from the Association for Cancer Research (l’ARC). RJ. Schneider was supported by grants from the Breast Cancer Research Foundation, the Avon Foundation for Women and the US. Department of Defense Breast Cancer Research Program. We thank Prof. N. Sonenberg for Wt and 4E-BP1−/−, 4E-BP2−/− MEFs, eIF4GI and eIF4GII antibodies.
References
- Sonenberg N, Morgan MA, Merrick WC, Shatkin AJ. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5′-terminal cap in mRNA. Proc Natl Acad Sci U S A 1978; 75:4843 - 7; http://dx.doi.org/10.1073/pnas.75.10.4843; PMID: 217002
- von der Haar T, Gross JD, Wagner G, McCarthy JE. The mRNA cap-binding protein eIF4E in post-transcriptional gene expression. Nat Struct Mol Biol 2004; 11:503 - 11; http://dx.doi.org/10.1038/nsmb779; PMID: 15164008
- Martineau Y, Azar R, Bousquet C, Pyronnet S. Anti-oncogenic potential of the eIF4E-binding proteins. Oncogene 2013; 32:671 - 7; http://dx.doi.org/10.1038/onc.2012.116; PMID: 22508483
- Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature 1990; 345:544 - 7; http://dx.doi.org/10.1038/345544a0; PMID: 2348862
- Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, et al. Dissecting eIF4E action in tumorigenesis. Genes Dev 2007; 21:3232 - 7; http://dx.doi.org/10.1101/gad.1604407; PMID: 18055695
- Furic L, Rong L, Larsson O, Koumakpayi IH, Yoshida K, Brueschke A, et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci U S A 2010; 107:14134 - 9; http://dx.doi.org/10.1073/pnas.1005320107; PMID: 20679199
- Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J 1997; 16:1909 - 20; http://dx.doi.org/10.1093/emboj/16.8.1909; PMID: 9155017
- Ueda T, Sasaki M, Elia AJ, Chio II, Hamada K, Fukunaga R, et al. Combined deficiency for MAP kinase-interacting kinase 1 and 2 (Mnk1 and Mnk2) delays tumor development. Proc Natl Acad Sci U S A 2010; 107:13984 - 90; http://dx.doi.org/10.1073/pnas.1008136107; PMID: 20679220
- Pyronnet S. Phosphorylation of the cap-binding protein eIF4E by the MAPK-activated protein kinase Mnk1. Biochem Pharmacol 2000; 60:1237 - 43; http://dx.doi.org/10.1016/S0006-2952(00)00429-9; PMID: 11007962
- Pyronnet S, Imataka H, Gingras AC, Fukunaga R, Hunter T, Sonenberg N. Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J 1999; 18:270 - 9; http://dx.doi.org/10.1093/emboj/18.1.270; PMID: 9878069
- Mader S, Lee H, Pause A, Sonenberg N. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol Cell Biol 1995; 15:4990 - 7; PMID: 7651417
- Hsieh AC, Costa M, Zollo O, Davis C, Feldman ME, Testa JR, et al. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer Cell 2010; 17:249 - 61; http://dx.doi.org/10.1016/j.ccr.2010.01.021; PMID: 20227039
- She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, Sasazuki T, et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell 2010; 18:39 - 51; http://dx.doi.org/10.1016/j.ccr.2010.05.023; PMID: 20609351
- Brunn GJ, Hudson CC, Sekulic A, Williams JM, Hosoi H, Houghton PJ, Lawrence JC, Jr., Abraham RT. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science (New York, NY 1997; 277:99-101.
- Svitkin YV, Gradi A, Imataka H, Morino S, Sonenberg N. Eukaryotic initiation factor 4GII (eIF4GII), but not eIF4GI, cleavage correlates with inhibition of host cell protein synthesis after human rhinovirus infection. J Virol 1999; 73:3467 - 72; PMID: 10074204
- Hou J, Lam F, Proud C, Wang S. Targeting Mnks for cancer therapy. Oncotarget 2012; 3:118 - 31; PMID: 22392765
- Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol 2004; 24:6539 - 49; http://dx.doi.org/10.1128/MCB.24.15.6539-6549.2004; PMID: 15254222
- Martineau Y, Azar R, Müller D, Lasfargues C, El Khawand S, Anesia R, et al. Pancreatic tumours escape from translational control through 4E-BP1 loss. Oncogene 2013; http://dx.doi.org/10.1038/onc.2013.100; PMID: 23563181
- Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, Wang X, Larsson O, Selvaraj A, Liu Y, Kozma SC, Thomas G, Sonenberg N. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science (New York, NY 2010; 328:1172-6.