Abstract
The human Mediator complex interacts extensively with the RNA polymerase II (Pol II) enzyme and recent data from our lab suggest activator-induced structural shifts within Mediator trigger activation of stalled Pol II. These results are discussed together with other recent findings regarding post-recruitment regulation of Pol II.
The discovery that RNA polymerase II (Pol II) occupies the 5′ end of uninduced heat shock genes in DrosophilaCitation1 coupled with similar observations at other loci in human cellsCitation2,Citation3 suggested that post-recruitment regulation of Pol II was important for proper transcriptional control. Since these studies, the significance of post-recruitment regulation of Pol II (i.e., positive or negative regulation of Pol II activity after its recruitment to the promoter) has become increasingly evident. Multiple studies in DrosophilaCitation4,Citation5 and human embryonic stem (ES) cellsCitation6,Citation7 have shown evidence of stalled Pol II at a majority of basal, activated or silent loci on a genome-wide scale, suggesting that post-recruitment regulation of Pol II activity is widespread. So what factors regulate activation of stalled Pol II complexes?
The process of transcription has historically been divided into three phases: initiation, elongation and termination. The C-terminal domain of the largest subunit of Pol II (Pol II CTD) has important functions in each of these phases. In mammals, the Pol II CTD consists of 52 heptad repeats of the general consensus YSPTSPS. The events leading to transcription start with a Pol II containing a hypo-phosphorylated CTD that assembles with the general transcription factors (GTFs) TFIIA, TFIIB, TFIID, TFIIE, TFIIF, TFIIH and Mediator at the gene promoter. During initiation, the CDK7 subunit within TFIIH phosphorylates the Pol II CTD at serine 5 (Ser5P). A common feature among stalled Pol II complexes is that initiation has halted 30–50 bases downstream of the transcription start site;Citation8 these stalled Pol II complexes also possess a Ser5-phosphorylated CTD.
Although we lack a complete understanding of how stalled Pol II is controlled, many of the main players have been determined. At genes with a stalled Pol II, the negative elongation factor NELF and DRB sensitivity-inducing factor DSIF act to halt Pol II with a 30–50 base transcript.Citation9 The positive transcription elongation factor b (P-TEFb) helps overcome negative regulation by NELF and DSIF. P-TEFb can phosphorylate many targets including the Pol II CTD at serine 2 (Ser2P), the Spt5 subunit of DSIF, and the NELF-E subunit within NELF. The kinase activity of P-TEFb is linked to a functional change in DSIF, the dissociation of NELF, the transition of Pol II from a stalled to an elongating state, and the accumulation of Ser2P CTD toward the 3′-end of the gene.Citation10 Recent data from Drosophila indicate DSIF can stably interact with Pol II that contains a short transcript; DSIF binding, in turn, appears essential for NELF binding.Citation11 The mechanisms whereby P-TEFb is recruited and activated appear to be more diverse and are discussed further below.
c-Myc, P-TEFb and the Activation of Stalled Pol II
Past studies have implicated the chromatin-binding protein Brd4 and transcription factors such as c-Myc, NFκB or nuclear receptors in the recruitment of P-TEFb to gene promoters containing a stalled polymerase (reviewed in ref. Citation10). In a recent genome-wide study, the Young lab found that 91% of Pol II-bound genes in mouse embryonic stem (mES) cells showed evidence of a stalled Pol II.Citation12 Additionally, they estimated that approximately 33% of actively transcribed genes in mES cells have promoters occupied by c-Myc. The c-Myc transcription factor is likely involved in recruiting P-TEFb to a number of c-Myc responsive genes.Citation13 Inhibiting c-Myc occupancy at these promoters (either pharmacologically or with shRNA knockdown) caused a decrease both in total Pol II and Pol II Ser2P within the coding regions of these genes, but had no effect on Pol II Ser5P or total Pol II at the promoter.Citation12 These results are consistent with c-Myc regulating the release of Pol II from a stalled state via the recruitment of P-TEFb. Interestingly, the kinase activity of P-TEFb appeared critical for Pol II release, as flavopiridol, which blocks P-TEFb kinase activity, had largely the same effects as c-Myc knockdown at c-Myc regulated genes.
p53-Directed Structural Shifts within Mediator Trigger Pol II Elongation
Although c-Myc may be acting to recruit P-TEFb to E box-containing promoters, c-Myc does not regulate all genes that possess stalled Pol II complexes. What then is the mechanism to release stalled Pol II at genes lacking c-Myc? Recent work from our lab supports a model in which structural changes within Mediator, caused by activator binding, might control transcriptional output at p53-responsive promoters. In particular, p53-induced shifts in Mediator structure appear to stimulate stalled Pol II complexes to elongate a transcript even in the absence of P-TEFb.Citation14
At 1.2 MDa, the multi-subunit Mediator complex acts as a physical and functional link between DNA-binding transcription factors and the general transcription machinery, including Pol II. Although it has no known enzymatic activity, Mediator appears to communicate regulatory signals from transcription factors to Pol II and the GTFs. Mediator is generally required for transcriptionCitation15 and interacts directly and extensively with the Pol II enzyme.Citation14,Citation16,Citation17 As many p53-responsive genes possess pre-loaded Pol II at their promoters in the absence of p53 activation,Citation3 we used p53 to study post-recruitment steps in transcription activation. We focused on two p53-regulated genes (p21 and HDM2) that had been shown previously to harbor stalled Pol II complexes.Citation18–Citation20
Using tiled ChIP analysis of the HDM2 and p21 loci in p53-null HCT116 cells, we found that the promoters of these p53-regulated genes contain pre-loaded Pol II, Mediator and the GTFs. As there was no p53 present in these cells, it was evident that p53 was not necessary for assembly of the initiation machinery at the HDM2 or p21 genes. Thus, Mediator occupying these promoters was not bound to p53. In this state, Mediator was unable to activate stalled Pol II: Pol II remained at the 5′ end of the gene and no mature transcripts were generated.Citation14
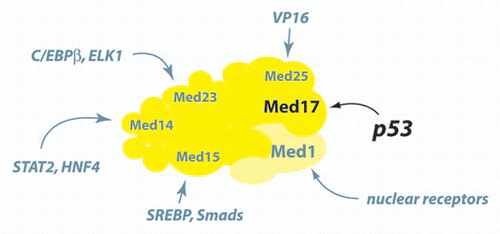
Two distinct p53 domains interact with two different subunits within the Mediator complex. The activation domain of p53 (p53AD) binds directly to the MED17 subunit of Mediator,Citation21 whereas the p53 C-terminal domain (p53CTD) binds the MED1 subunit.Citation14 Expression of WT p53 or various p53 mutants in p53-null HCT116 cells enabled us to probe which domains within p53 were necessary to activate stalled Pol II at HDM2 and p21. Interestingly, WT p53, a truncated p53 lacking the CTD (p53ΔCTD) or an oncogenic p53AD mutant (p53QS) showed similar Mediator, Pol II and GTF occupancy at p21 or HDM2. WT p53 and p53ΔCTD, however, caused the overall Pol II ChIP signal to extend throughout the gene and increased the Pol II Ser2P signal towards the 3′ end of the gene. By contrast, the p53QS mutant did not. These results suggest that, on its own, the p53CTD interaction with Mediator is not transcriptionally productive; also, the p53AD-Mediator interaction (present within WT p53 and p53ΔCTD) appears critical for activating stalled Pol II at HDM2 and p21.
The cell-based ChIP and RT-qPCR data were confirmed and further developed in vitro using immobilized template assays on synthetic p53 promoters as well as with chromatin sedimentation assays performed with purified components on the native HDM2 promoter. These in vitro studies revealed again that WT p53 was not required for Pol II recruitment, as mutant p53 proteins lacking either a functional AD (p53QS) or missing the CTD (p53ΔCTD) were each capable of assembling the initiation machinery at the promoter. Despite similar Pol II, Mediator and GTF promoter occupancy, in vitro transcription assays revealed that only p53 containing a functional AD could elicit Pol II elongation. In fact, transcripts of only 155 bases would have been detected in these assays, clearly demonstrating that Pol II did not transition to productive elongation in the absence of a functional p53AD. Notably, the reconstituted, in vitro transcription system did not contain P-TEFb, indicating that promoter-bound Pol II was being activated to elongate by a mechanism that did not require P-TEFb.
Mediator is known to alter its conformational state upon activator binding and distinct Mediator structural states may help coordinate gene-specific activation mechanisms.Citation22 Interestingly, p53AD, which is generally required to activate expression of p53 target genes,Citation23–Citation25 induced a structural shift within Mediator that was distinct from Mediator bound only to the p53CTD.Citation14 This p53AD-Mediator structural state also differed from the structure of Mediator without an activator bound (the so-called unliganded Mediator structure). In the case of p53AD-Mediator, a large pocket domain within Mediator was clearly evident, whereas this domain was absent within the transcriptionally-inactive p53CTD-Mediator structure. In fact, instead of the large pocket domain, a wall of protein density was present within the p53CTD-Mediator structure. Thus, formation of the pocket domain, which corresponds to the Pol II binding site within Mediator, correlates with activation of stalled Pol II. Further supporting this, WT p53 tetramers also induced formation of the pocket domain upon binding Mediator. Collectively, the cell-based, in vitro and structural data suggest that Mediator structural shifts—induced by p53 binding—allow Pol II to bypass promoter-proximal stalling and transition to a productively-elongating state.
Interestingly, the large pocket domain induced by p53-Mediator binding is also observed in Mediator bound to other activation domains (including VP16, SREBP and several nuclear receptors). By contrast, transcriptionally inactive versions of the Mediator complex, such as p53CTD-Mediator, unliganded Mediator or Mediator bound to the CDK8 module each contain protein density that occludes the pocket domain,Citation15 suggesting that structural shifts within Mediator control fundamental events in transcription initiation and elongation.
Do Mediator and P-TEFb Act Independently or Cooperatively to Regulate Stalled Pol II?
It is clear that multiple mechanisms are in place to control Pol II elongation in gene-specific ways. For example, the chromatin-targeting Brd4 protein contributes to P-TEFb activation and appears to promote Mediator association with P-TEFb, suggesting a cooperativity between Mediator and P-TEFb in the presence of Brd4.Citation26 As another example, the p21 gene is not dependent on P-TEFb kinase activity to overcome Pol II stalling at its promoter. In fact, the P-TEFb inhibitor DRB actually induces p21 transcriptionCitation27 signifying that at p21 there exists a P-TEFb independent mechanism to overcome stalled Pol II. Based upon our recent work, p53-directed structural shifts within Mediator appear important for activating Pol II elongation at p21 and HDM2 in the absence of P-TEFb.Citation14 Whether the activation of stalled Pol II via P-TEFb also involves Mediator, or whether Mediator might act through a mechanism that is completely independent of P-TEFb remains to be seen. Whereas in vitro transcription assays indicated a structure/function link between p53-Mediator and Pol II elongation in the absence of P-TEFb, it is important to note that auxiliary factors such as DSIF and NELF were also absent in these assays. Thus, whereas MediatorCitation28,Citation29 and activator-induced structural shiftsCitation14 are clearly implicated in post-recruitment activation of transcription, it is likely that Mediator functions coordinately with P-TEFb and other factors to strictly enforce Pol II stalling and activation. Supporting this, the Gilmour and Price labs have noted that DSIF and NELF are insufficient to stabilize a stalled Pol II complex: additional factors are required.Citation11,Citation30
Another potential link between Mediator and P-TEFb involves the CDK8 module (consisting of CDK8, Cyclin C, MED12 and MED13), which can reversibly associate with Mediator to control its function. The free CDK8 module as well as CDK8-Mediator associate with P-TEFb.Citation31,Citation32 Because this interaction occurs without any detectable c-Myc, the CDK8 module could help direct P-TEFb to activate Pol II elongation at genes that are not regulated by c-Myc. Indeed, the CDK8 module and P-TEFb appear to cooperate in activating Pol II elongation at serum response genes in HCT116 cells.Citation31 In addition, human CDK8-Mediator is capable of phosphorylating histone H3S10 in a chromatin context,Citation33 and H3S10 phosphorylation has been linked to P-TEFb occupancy at transcriptionally active genes. Knockdown of the H3S10-specific JIL-1 kinase in Drosophila resulted in a failure to induce Hsp70 expression and this correlated with a loss of P-TEFb, but not NELF or DSIF, from Hsp70 promoters after heat shock.Citation34 In human cells, depletion of PIM1, another H3S10 kinase, blocked Pol II elongation at c-FosL1 and ID2.Citation35 Given that CDK8-Mediator can phosphorylate H3S10 in human cells, CDK8 kinase activity might contribute to activation of Pol II elongation, perhaps in concert with P-TEFb.
Mediator could also be functioning cooperatively with c-Myc as well. Several reports have suggested c-Myc interacts with Mediator.Citation36,Citation37 Furthermore, c-Myc binds Transformation/Transcription domain-Associated Protein (TRRAP),Citation38 which exists as part of the human STAGA complexCitation39 but is also known to independently associate with Mediator.Citation40 TRRAP-associated Mediator complexes contain the CDK8 module and the acetyltransferase GCN5L. Because they contain the CDK8 module, these TRRAP-containing “T/G Mediator” complexes are capable of phosphorylating H3S10 on chromatin.Citation40 Taken together, these observations provide a link between c-Myc, Mediator and H3S10 phosphorylation that might facilitate P-TEFb-dependent activation of Pol II elongation at a subset of c-Myc-regulated genes. Further mechanistic studies will be required to explore this possibility.
Mediator as a Therapeutic Target?
Because a subset of p53-responsive genes have the transcription initiation machinery pre-loaded at their promoters in the absence of p53,Citation3,Citation14 it is plausible that converting Mediator into a p53-bound structural state could artificially activate Pol II elongation in cancer cells with non-functioning p53. Generating a pharmacological agent capable of mimicking the p53AD-MED17 interaction might trigger the structural shift within Mediator that releases stalled Pol II from select p53-responsive genes. Transient reactivation of p53 has been shown to cause tumor regression in a number of studies.Citation41–Citation43 The Jacks group found that apoptosis in lymphomas and cellular senescence in sarcomas led to tumor regression.Citation43 The Lowe lab observed that transient p53 activation triggered senescence in liver tumor cells, and this p53-dependent senescence elicited an innate immune response that served to clear the tumor.Citation42 Thus, tumor regression was induced by activating either the apoptosis or the cell cycle arrest arms of the p53 pathway. Although the p53AD interaction surface with MED17 remains to be characterized, it may be feasible to target this surface with small molecules. And because other transcription factors target different surfaces within Mediator (see ), a small molecule that mimics p53AD binding to MED17 might selectively activate p53 responsive genes while leaving non-p53 target genes unaffected.
References
- Gilmour DS, Lis JT. RNA polymerase II interacts with the promoter region of the noninduced hsp70 gene in Drosophila melanogaster cells. Mol Cell Biol 1986; 6:3984 - 3989
- Sawado T, Halow J, Bender MA, Groudine M. The beta-globin locus control region (LCR) functions primarily by enhancing the transition from transcription initiation to elongation. Genes Dev 2003; 17:1009 - 1018
- Espinosa JM, Verdun RE, Emerson B. p53 functions through stress- and promoter-specific recruitment of transcription initiation components before and after DNA damage. Mol Cell 2003; 12:1015 - 1027
- Muse GW, Gilchrist DA, Nechaev S, Shah R, Parker JS, Grissom SF, et al. RNA polymerase is poised for activation across the genome. Nat Genet 2007; 39:1507 - 1511
- Zeitlinger J, Stark A, Kellis M, Hong JW, Nechaev S, Adelman K, et al. RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo. Nat Genet 2007; 39:1512 - 1516
- Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell 2007; 130:77 - 88
- Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 2008; 322:1845 - 1848
- Lee C, Li X, Hechmer A, Eisen M, Biggin MD, Venters BJ, et al. NELF and GAGA factor are linked to promoter-proximal pausing at many genes in Drosophila. Mol Cell Biol 2008; 28:3290 - 3300
- Nechaev S, Fargo DC, dos Santos G, Liu L, Gao Y, Adelman K. Global analysis of short RNAs reveals widespread promoter-proximal stalling and arrest of Pol II in Drosophila. Science 2010; 327:335 - 338
- Peterlin BM, Price DH. Controlling the elongation phase of transcription with P-TEFb. Mol Cell 2006; 23:297 - 305
- Missra A, Gilmour DS. Interactions between DSIF (DRB sensitivity inducing factor), NELF (negative elongation factor) and the Drosophila RNA polymerase II transcription elongation complex. Proc Natl Acad Sci USA 2010; 107:11301 - 11306
- Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, et al. c-Myc regulates transcriptional pause release. Cell 2010; 141:432 - 445
- Eberhardy SR, Farnham PJ. c-Myc mediates activation of the cad promoter via a post-RNA polymerase II recruitment mechanism. J Biol Chem 2001; 276:48562 - 48571
- Meyer KD, Lin S, Bernecky C, Gao Y, Taatjes DJ. p53 activates transcription by directing structural shifts in Mediator. Nat Struct Mol Biol 2010; 17:753 - 760
- Taatjes DJ. The human Mediator complex: a versatile, genome-wide regulator of transcription. Trends Biochem Sci 2010; 35:315 - 322
- Davis JA, Takagi Y, Kornberg RD, Asturias FA. Structure of the yeast RNA polymerase II holoenzyme: Mediator conformation and polymerase interaction. Mol Cell 2002; 10:409 - 415
- Naar AM, Taatjes DJ, Zhai W, Nogales E, Tjian R. Human CRSP interacts with RNA polymerase II CTD and adopts a specific CTD-bound conformation. Genes Dev 2002; 16:1339 - 1344
- Li P, Yao H, Zhang Z, Li M, Luo Y, Thompson PR, et al. Regulation of p53 target gene expression by peptidylarginine deiminase 4. Mol Cell Biol 2008; 28:4745 - 4758
- Glover-Cutter K, Kim S, Espinosa JM, Bentley DL. RNA polymerase II pauses and associates with pre-mRNA processing factors at both ends of genes. Nat Struct Mol Biol 2008; 15:71 - 78
- Donner AJ, Szostek S, Hoover JM, Espinosa JM. CDK8 is a stimulus-specific positive coregulator of p53 target genes. Mol Cell 2007; 27:121 - 133
- Ito M, Yuan C, Malik S, Gu W, Fondell JD, Yamamura S, et al. Identity between TRAP and SMCC complexes indicates novel pathways for the function of nuclear receptors and diverse mammalian activators. Mol Cell 1999; 3:361 - 370
- Taatjes DJ, Naar AM, Andel F, Nogales E, Tjian R. Structure, function and activator-induced conformations of the CRSP coactivator. Science 2002; 295:1058 - 1062
- Jimenez GS, Nister M, Stommel JM, Beeche M, Barcarse EA, Zhang XQ, et al. A transactivation-deficient mouse model provides insights into Trp53 regulation and function. Nat Genet 2000; 26:37 - 43
- Johnson TM, Hammond EM, Giaccia A, Attardi LD. The p53QS transactivation-deficient mutant shows stress-specific apoptotic activity and induces embryonic lethality. Nat Genet 2005; 37:145 - 152
- Lin J, Chen J, Elenbaas B, Levine AJ. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55 kD protein. Genes Dev 1994; 8:1235 - 1246
- Yang Z, Yik JHN, Chen R, He N, Jang MK, Ozato K, et al. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell 2005; 19:535 - 545
- Gomes NP, Bjerke G, Llorente B, Szostek SA, Emerson BM, Espinosa JM. Gene-specific requirement for P-TEFb activity and RNA polymerase II phosphorylation within the p53 transcriptional program. Genes Dev 2006; 20:601 - 612
- Malik S, Wallberg AE, Kang YK, Roeder RG. TRAP/SMCC/mediator-dependent transcriptional activation from DNA and chromatin templates by orphan nuclear receptor hepatocyte nuclear factor 4. Mol Cell Biol 2002; 22:5626 - 5637
- Wang G, Balamotis MA, Stevens JL, Yamaguchi Y, Handa H, Berk AJ. Mediator requirement for both recruitment and postrecruitment steps in transcription initiation. Mol Cell 2005; 17:683 - 694
- Renner DB, Yamaguchi Y, Wada T, Handa H, Price DH. A highly purified RNA polymerase II elongation control system. J Biol Chem 2001; 276:42601 - 42609
- Donner AJ, Ebmeier CC, Taatjes DJ, Espinosa JM. CDK8 is a positive regulator of transcriptional elongation within the serum response network. Nat Struct Mol Biol 2010; 17:194 - 201
- Ebmeier CC, Taatjes DJ. Activator-Mediator binding regulates Mediator-cofactor interactions. Proc Natl Acad Sci USA 2010; 107:11283 - 11288
- Knuesel MT, Meyer KD, Donner AJ, Espinosa JM, Taatjes DJ. The human CDK8 subcomplex is a histone kinase that requires Med12 for activity and can function independently of Mediator. Mol Cell Biol 2009; 29:650 - 661
- Ivaldi MS, Karam CS, Corces VG. Phosphorylation of histone H3 at Ser10 facilitates RNA polymerase II release from promoter-proximal pausing in Drosophila. Genes Dev 2007; 21:2818 - 2831
- Zippo A, De Robertis A, Serafini R, Oliviero S. PIM1-dependent phosphorylation of histone H3 at serine 10 is required for MYC-dependent transcriptional activation and oncogenic transformation. Nat Cell Biol 2007; 9:932 - 944
- Eberhardy SR, Farnham PJ. Myc recruits P-TEFb to mediate the final step in the transcriptional activation of the cad promoter. J Biol Chem 2002; 277:40156 - 40162
- Bouchard C, Marquardt J, Bras A, Medema RH, Eilers M. Myc-induced proliferation and transformation require Akt-mediated phosphorylation of FoxO proteins. EMBO J 2004; 23:2830 - 2840
- McMahon SB, Van Buskirk HA, Dugan KA, Copeland TD, Cole MD. The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins. Cell 1998; 94:363 - 374
- Martinez E, Kundu TK, Fu J, Roeder RG. A human SPT3-TAFII31-GCN5-L acetylase complex distinct from transcription factor IID. J Biol Chem 1998; 273:23781 - 23785
- Meyer KD, Donner AJ, Knuesel M, York AG, Espinosa JM, Taatjes DJ. Cooperative activity of CDK8 and GCN5L within Mediator directs tandem phosphoacetylation of histone H3. EMBO J 2008; 27:1447 - 1457
- Martins CP, Brown-Swigart L, Evan GI. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell 2006; 127:1323 - 1334
- Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, et al. Restoration of p53 function leads to tumour regression in vivo. Nature 2007; 445:661 - 665
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007; 445:656 - 660