Abstract
The spatial organization of the genome is known to be important for regulation of gene transcription during normal development and in disease states. However, molecular mechanisms governing structural rearrangements in the genome are still poorly understood. Recently, a role for transcription factors in reorganization of the three-dimensional genome structure has been suggested. Distribution of Signal Transducer and Activator of Transcription (STAT) binding sites on genomic DNA and the ability of this family of transcription factors to form phosphorylated (P-STAT) tetramers and unphosphorylated (U-STAT) dimers suggest that some family members, particularly STAT3, may be directly involved in regulation of chromatin topology. The hypothesis is supported by observations of binding of P-STAT3 dimers to adjacent sites on DNA and ability of U- STAT3 to bend and loop DNA. Here we discuss potential roles for STAT3 and other STAT family members in the regulation of gene expression by modulation of chromatin organization.
An organism’s development and its interactions with the environment depend on precise spatio-temporal regulation of gene expression programs that are achieved through the interplay of the epigenetic landscape, the presence of cofactors and binding of transcription factors (TF), and the dynamic localization of chromatin regions to shared nuclear compartments.Citation1-Citation3 The three-dimensional (3D) structure of the genome is not random and, although not completely understood, it is known to play an important role in gene expression during normal development and in diseases such as cancer.Citation2,Citation3 Mounting evidence suggests that genomes are organized into higher-order chromatin domains and that transcription factors mediate interactions between distant chromatin regions through formation of looped structures resulting in global changes in the 3D structure of chromosomes and coordinated transcription of target genes.Citation1-Citation5 Similarly, it has been observed that oncogenic transcription factors, such as androgen receptor (AR), estrogen receptor (ER), and early growth response factor (EGR), that bind to thousands of sites in the genome without a clear transcriptional role on nearby genes could still play a role by restructuring the shape of the genome.Citation5-Citation8 In this report, we hypothesize that Signal Transducer and Activator of Transcription (STAT) family members may regulate gene expression and contribute to oncogenic processes via regulation of chromatin 3D structure. We summarize the evidence for potential chromatin-organizing function of phosphorylated and unphosphorylated STAT proteins, to justify the need for further investigations.
Mammalian cells express seven STAT proteins: STAT1, 2, 3, 4, 5A, 5B and 6, that transduce extracellular signals to the nucleus and regulate transcription of target genes.Citation9 STAT proteins were previously considered as latent cytoplasmic transcription factors activated by tyrosine phosphorylation in response to various extracellular signals, such as growth factors, hormones, and cytokines.Citation9 When these ligands bind to their cognate receptors, one or more STAT proteins become activated by JAK-mediated tyrosine phosphorylation of a critical tyrosine residue and form a dimer (P-STAT) through reciprocal Src homology 2-phosphotyrosine (SH2-pTyr) interaction. The dimeric P-STAT proteins translocate to the nucleus, where they bind to consensus STAT binding sequences, so called IFNgamma activated sequence (GAS motif), and thereby activate transcription of their target genes.Citation9 Although STATs recognize similar, often identical, DNA sequences, their involvement in such processes as cell differentiation, proliferation, apoptosis, hematopoiesis, immunity, inflammation, and development are different.Citation10 In many cancerous cells, with dysregulated growth factor signaling, STAT proteins, in particular STAT3, are constitutively activated by tyrosine phosphorylation and drive expression of genes that enhance proliferation, angiogenesis, invasion, and suppression of apoptosis.Citation10
It has been proposed that two P-STAT dimers bound to adjacent GAS elements may form a P-STAT tetramer via N-terminal domain (ND) interaction.Citation11-Citation15 Such cooperativity in DNA binding via NDs allows fine-tuning of transcriptional responses through selective binding of different STAT proteins on the promoters containing multiple STAT binding sites and through enhanced binding to weak STAT-binding sites. So far, it has been established that the ND of STAT1, STAT4, STAT5 and STAT3 are necessary for tetramer formation, at least on selected promoters.Citation14 ND-mediated P-STAT5 tetramer was found to be essential for IL-2-induced regulation of the IL-2 response element in the human IL-2Ra gene, while P-STAT3 tetramer appears to be important for an IL-6-inducible activation of α2-macroglobulin gene expression.Citation11 Although functional importance of tetramer formation was revealed by the decreased levels of transcriptional activation associated with hypomorphic mutations in ND residues, the mechanism, by which STAT tetramerization contributes to the regulation of gene expression, remains elusive.Citation16
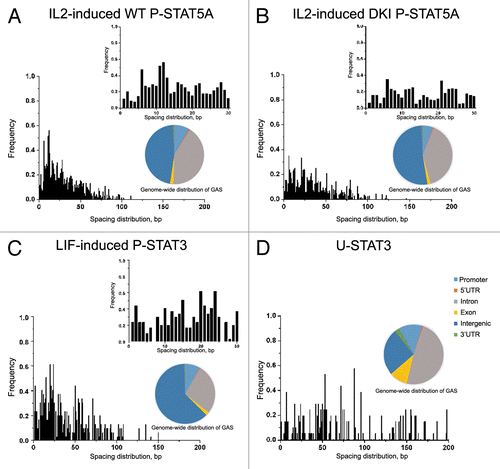
A recent study elucidated the differences in DNA binding between phosphorylated STAT5 dimers and tetramers by generating STAT5A-STAT5B double knockin (DKI) ND mutant mice that lack P-STAT5 tetramers.Citation17 By studying IL-2-induced gene expression in DKI T cells vs. wild-type (WT) T cells and using ChIP-seq methodology to identify genome-wide binding sites for P-STAT5A/B dimer and tetramer, Lin et al. have been able to establish the consensus binding motifs for P-STAT5A/B, including the optimal spacing between two GAS motifs located within 30 nucleotides for tetramer binding.Citation17 We re-analyzed the data set for GAS distribution at longer distances,Citation17 and also performed similar analysis of LIF-induced STAT3 binding to DNA,Citation18 to determine the optimal spacing between neighboring GAS sites using the CisGenome programCitation19 that is widely used for tiling array, ChIP-seq and genome analysis. Since the profiles of ChIP-seq or ChIP-chip data are often noisy and diffusive, spanning regions ranging from short DNA fragments wrapped around several nucleosomes to large DNA domains covering multiple genes, we first narrowed down the diffusive regions to ChIP-enriched sequences and then mapped out location of the GAS-like motif (T/C)(T/A)(C/T)(T/C)N(A/G/T)G(A/T)A in the ChIP-enriched data. The spacing distributions between the GAS-like motifs for WT STAT5A/B binding agree with the previously published findings that the binding is most favored when two adjacent GAS motifs are separated by about 11–12 bp (). However, this spacing of 11–12 bp is absent in DKI STAT5A/B binding (). Since DKI STAT5A/B prohibits tetramer formation, this observation suggests that the P-STAT5A/B tetramers bind to GAS-like motifs with an optimal spacing of 11–12 bp. Analysis of STAT3 data set in which phosphorylation of STAT3 was induced by LIF, showed the spacing distribution between two GAS-like motifs with significant peaks in short distances of 20 bp and 24 bp (). The distribution of the optimal spacing between GAS sites for P-STAT3 and WT P-STAT5A/B are similar with high correlation coefficients of 0.78 and 0.76 respectively. These results further indicate that binding distributions of the P-STAT proteins are similar and their tetramer binding may play an important role in the regulation of gene expression.
Figure 1. Analysis of ChIP-seq and ChIP-chip data reveals differences in spacing distributions of GAS-like motifs for different forms of STAT proteins. (A) IL-2 induced WT P-STAT5A favors two GAS motifs separated by 11–12 bp (mm8 data set, GSE36890), while this spacing of 11–12 bp is absent for DKI P-STAT5A that prohibits tetramer formation in mouse T cells (B). (C) Analysis of LIF-induced P-STAT3 binding in mouse ES cells shows the spacing distribution between two GAS-like motifs with significant peaks at 20 bp and 24 bp (mm8 data set, GSM288353). (D) Analysis of STAT3 binding in DU145 prostate cancer cells with low levels of P-STAT3 shows the spacing distribution peaked at about 50 bp, 70 bp, 90 bp, and 140 bp (hg18 data set, GSE25866). Genome-wide distributions of GAS binding sites bound by various STATs indicate that only 8–12% of all GAS sites are located in the promoter regions with the majority in introns and intergenic regions for both mouse genome (mm8) and human genome (hg18).
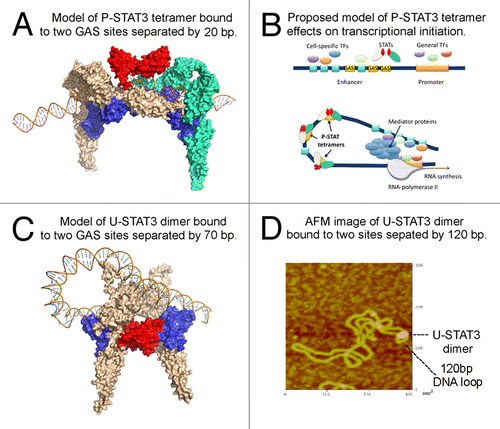
The binding of P-STAT tetramer leads to an amplified or super-additive effect on activating transcription.Citation11-Citation15,Citation17 To better understand how tetramer may be involved in regulation of gene expression, we modeled the interactions of two P-STAT3 dimers bound to two adjacent GAS motifs (). We have built the STAT3 protein model using protein structure and function prediction program, I-TASSER.Citation20 The DNA-protein complex structures have been constructed by 3dRNACitation21 and 3dRPCCitation22 programs that are designed for structural modeling and docking of nucleic acid structures. While the two NDs within a P-STAT dimer are separated and do not interact with each other,Citation23 the NDs of two P-STAT dimers can interact to form a tetramer.Citation24 Unexpectedly, we found that in order to be simultaneously bound by two dimers, the DNA fragments containing two GAS elements need to bend. shows the structure of P-STAT3 tetramer. The two dimers (colored in wheat and green, respectively) are linked via ND-ND interaction. The DNA should bend (with bending angles about 140–150 degrees) to thread through two phosphorylated dimers. These modeling data lead us to propose that the binding of P-STAT tetramer may direct the bending of an otherwise straight DNA, facilitating and even determining proximal and distal DNA-protein and protein-protein contacts involved in various steps leading to transcription initiation ().
Figure 2. STAT3 may regulate chromatin topology through bending and looping of DNA. (A) Modeled structure of phosphorylated STAT3 tetramer bound to DNA. Protein structure was generated using I-TASSER. The DNA-protein complex structures are constructed using 3dRNA and 3dRPC programs designed for structural modeling and docking of nucleic acid structures. The two dimers (colored in wheat and green, respectively) are linked via ND-ND interaction (colored in red) and they bind to DNA via DBD (colored in blue). (B) Proposed mechanism by which P-STAT3 tetramer may regulate gene expression through DNA bending. General transcription factors bind to promoter region, while tissue-specific and/or inducible transcription factors bind to their cognate binding sites within an enhancer sequence. In order for enhanceosome to be assembled, these transcriptional factors need to be positioned in the optimal way. This may be achieved through DNA bending caused by DNA-bending proteins, in particular P-STAT3 tetramer (or a series of P-STAT3 tetramers), to promote cooperation between various proteins bound to distant DNA regions. Once all players are interacting with each other, mediator complex, and RNA-polymerase II, the transcription is initiated. (C) Modeled structure of U-STAT3 dimer bound to two DNA sites separated by 70 bp suggests that U-STAT3 dimer may regulate gene expression through DNA looping. The STAT3 ND is colored in red and the DBD is colored in blue. (D) Atomic force microscopy image of U-STAT3 dimer bound to DNA forming a loop of about 120 bp. The data was generated using characterized supercoiled pUC8F14C plasmid and purified full-length recombinant unphosphorylated STAT3.
Our current understanding of transcriptional regulation implies that binding of multiple regulatory proteins to their binding sites on an enhancer sequence can catalyze the formation of an enhanceosome, a large protein complex that acts synergistically to activate transcription.Citation25 However, in order for transcription to be highly effective, all necessary factors have to be present and optimally positioned. This is achieved, at least in part, through protein-induced DNA bending to promote cooperation between various proteins bound to a promoter and an enhancer regions (). Indirect evidence in support of STAT’s role in the DNA bending function is offered the location of GAS elements within the genome. Analysis of STAT5 and STAT3 binding sites shows that only 8–10% are located in proximal promoters, while the majority are found in the intergenic regions or introns (), where enhancers are located. This is in agreement with a recent study demonstrating that STAT3 occupies both typical enhancers and super-enhancers that consist of clusters of enhancers that are densely occupied by the master regulators and Mediator.Citation26 Based on the analysis of binding sites and STAT tetramer interactions with DNA we hypothesize that enhanced activation of gene expression by tetrameric STAT proteins relies on the DNA bending function, thus allowing for interactions among several transcription factors bound to enhancer and promoter regions. Furthermore, a recent report on striking global reorganization of the nuclear architecture that occurs in naive T cells as they take on one of the two distinct fates, Th1 or Th2, suggested that the lineage-inducing transcription factors, STAT proteins, play a critical role in specifying such a functional nuclear architecture through their direct association with genic and non-genic loci.Citation27 These data provide a basis for understanding how STAT transcription factors not only regulate gene expression but also may play a role in shaping a nuclear architecture that supports specialized effector function in cellular differentiation.Citation27
We propose that ND interactions may have a role in 3D chromatin organization not only via P-STAT tetramerization, but also through ND-dependent dimerization of some U-STAT proteins, in particular for STAT3. Recently, roles for unphosphorylated STAT proteins in regulation of gene expression have been reported, based on their ability to shuttle between cytoplasm and nucleus, to bind DNA and activate or suppress gene transcription, and even to maintain stability of heterochromatin.Citation16 Moreover, Atomic Force Microscopy (AFM) imaging shows that U-STAT3 may bind to DNA both as a dimer and as a monomer.Citation28 Molecular modeling data for U-STAT3 interactions with DNA suggests a potential mechanism for an U-STAT3 dimer-mediated DNA bridging/looping (). Structure of the P-STAT3 dimer reveals that its formation allows both DNA-binding domains (DBD) to come sufficiently close to each other to interact with two halves of the same palindromic GAS site.Citation23 On the contrary, the dimer of U-STAT3 is formed via interactions of the ND, and, therefore, DBDs in such dimers are positioned in a way that only one of two DBDs can interact with the same DNA site (). It has been shown that STAT1, a close homolog of STAT3, contacts only one-half of a palindromic GAS motif when binds DNA as unphosphorylated monomer.Citation29 Therefore, DNA binding domains of the U-STAT3 dimers are facing the opposite directions, and potentially, the DBD of the second STAT3 molecule is available for interaction with a DNA site that is separated from a first site at least by 50Å (). Molecular modeling data suggest that in order to be bound by both DBDs of U-STAT3 dimer, DNA needs to form a loop ().
To evaluate whether the distribution of binding sites for U-STAT3 supports this hypothesis, we re-analyzed the STAT3 ChIP-chip (hg18) data set.Citation30 We have previously characterized STAT3 chromatin binding sites in human prostate cancer cells DU145 that have some levels of P-STAT3,Citation30 and found that in these cells both P-STAT3 and U-STAT3 were directly involved in regulation of gene expression.Citation30 Remarkably, the distributions of GAS sites that are preferentially bound by U-STAT3Citation30 are very different from those for cytokine-induced WT P-STAT5 and P-STAT3 binding with correlation values 0.29 and 0.25, respectively. We observed peaks with longer spacing, in particular for the distances of 50–70 bp, 110–140 bp, 170 bp, 200–220 bp (). The differences in the spacing distributions are likely to reflect binding of U-STAT3 dimers to the sites distant to each other. Two peaks with the shorter distances of 10 bp and 20 bp may reflect some level of P-STAT3 tetramer binding to adjacent sites (). We interpret these data as that longer distance between binding sites support a potential role for U-STAT3 dimers in DNA looping and transcriptional regulation through changes in chromatin structure. Furthermore, our previous observation has shown that U-STAT3 binds to the 4-way junction region and DNA nodes, similar to the DNA looping mechanism induced by MAR proteins.Citation28 It is tempting to speculate that the ability of U-STAT3 to bind and bend/loop DNA () by bringing together distant DNA regions and other proteins may underlie the mechanism by which STAT3 contributes to chromatin organization and potentially explain both activating and suppressing effects of this protein on gene expression.
In addition, the reports by Shi et al. on non-canonical JAK-STAT signaling in Drosophila demonstrated that dissociation of U-STAT from DNA in response to cytokine signaling triggers changes in chromatin dynamics.Citation31,Citation32 To date, there is little evidence for this mechanism in mammalian cells, although a role for STAT family members in heterochromatin maintenance in mammalian cells has been suggested.Citation33 Our data also underline an important role of the ND in STAT signaling and suggest that inhibition of the ND may affect U-STAT dimerization and P-STAT tetramerization and prevent/inhibit DNA looping/bending leading to changes in 3D chromatin structure and gene expression. Indeed, when using inhibitor of the STAT3 ND, we observed changes in heterochromatin foci localization in the nucleus,Citation30 further suggesting that STAT3 may be involved in the formation of higher order protein-DNA or protein-protein complexes responsible for regulation of 3D chromatin structure in the nucleus. However, the exact mechanisms are still to be investigated and further studies using conformational chromatin immunoprecipitation assay are needed to better understand how binding of P-STAT3 tetramers or U-STAT3 dimers may induce DNA bending and/or looping and bring together distal regulatory regions to induce gene expression.
In summary, the analysis presented in this paper allows us to propose a novel mechanism by which phosphorylated and unphosphorylated STAT3 and possibly other STAT proteins may regulate gene expression. The anticipated ability of P-STAT3 tetramers to bend DNA and U-STAT3 dimers to bring together distant DNA regions for DNA looping warrants further investigations as a potentially important, and thus far overlooked, mechanism by which STAT3 controls chromatin organization and gene expression. The proposed new function of STAT3 also emphasizes an important role of the NDs in STAT signaling. It suggests that inhibition of the ND may affect U-STAT dimerization and P-STAT tetramerization,Citation34 thus preventing/inhibiting DNA looping/bending leading to changes in 3D chromatin structure and gene expression. Given the role STAT proteins play in regulation of immune responses, inflammation and cancer, the significance of further studies into architectural function of STAT proteins lies in discovering novel mechanisms by which STATs may regulate gene expression and designing novel approaches for manipulating them for therapeutic applications.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research was supported in part by American Cancer Society Grant IRG 97–152–17 (OT); NSF grant 0941228 and GW CCFF award (YZ and CZ); the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (NT); and National Cancer Institute Award P30CA051008.
References
- Sexton T, Umlauf D, Kurukuti S, Fraser P. The role of transcription factories in large-scale structure and dynamics of interphase chromatin. Semin Cell Dev Biol 2007; 18:691 - 7; http://dx.doi.org/10.1016/j.semcdb.2007.08.008; PMID: 17950637
- Sexton T, Schober H, Fraser P, Gasser SM. Gene regulation through nuclear organization. Nat Struct Mol Biol 2007; 14:1049 - 55; http://dx.doi.org/10.1038/nsmb1324; PMID: 17984967
- Osborne CS, Chakalova L, Brown KE, Carter D, Horton A, Debrand E, Goyenechea B, Mitchell JA, Lopes S, Reik W, et al. Active genes dynamically colocalize to shared sites of ongoing transcription. Nat Genet 2004; 36:1065 - 71; http://dx.doi.org/10.1038/ng1423; PMID: 15361872
- Sexton T, Bantignies F, Cavalli G. Genomic interactions: chromatin loops and gene meeting points in transcriptional regulation. Semin Cell Dev Biol 2009; 20:849 - 55; http://dx.doi.org/10.1016/j.semcdb.2009.06.004; PMID: 19559093
- Elemento O, Rubin MA, Rickman DS. Oncogenic transcription factors as master regulators of chromatin topology: a new role for ERG in prostate cancer. Cell Cycle 2012; 11:3380 - 3; http://dx.doi.org/10.4161/cc.21401; PMID: 22918253
- Itkonen H, Mills IG. Chromatin binding by the androgen receptor in prostate cancer. Mol Cell Endocrinol 2012; 360:44 - 51; http://dx.doi.org/10.1016/j.mce.2011.09.037; PMID: 21989426
- Liu MH, Cheung E. Estrogen receptor-mediated long-range chromatin interactions and transcription in breast cancer. Mol Cell Endocrinol 2013; PMID: 24071518
- Theodorou V, Carroll JS. Estrogen receptor action in three dimensions - looping the loop. Breast Cancer Res 2010; 12:303; http://dx.doi.org/10.1186/bcr2470; PMID: 20156333
- Stark GR, Darnell JE Jr.. The JAK-STAT pathway at twenty. Immunity 2012; 36:503 - 14; http://dx.doi.org/10.1016/j.immuni.2012.03.013; PMID: 22520844
- O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med 2013; 368:161 - 70; http://dx.doi.org/10.1056/NEJMra1202117; PMID: 23301733
- Zhang X, Darnell JE Jr.. Functional importance of Stat3 tetramerization in activation of the alpha 2-macroglobulin gene. J Biol Chem 2001; 276:33576 - 81; http://dx.doi.org/10.1074/jbc.M104978200; PMID: 11438543
- Vinkemeier U, Moarefi I, Darnell JE Jr., Kuriyan J. Structure of the amino-terminal protein interaction domain of STAT-4. Science 1998; 279:1048 - 52; http://dx.doi.org/10.1126/science.279.5353.1048; PMID: 9461439
- Vinkemeier U, Cohen SL, Moarefi I, Chait BT, Kuriyan J, Darnell JE Jr.. DNA binding of in vitro activated Stat1 alpha, Stat1 beta and truncated Stat1: interaction between NH2-terminal domains stabilizes binding of two dimers to tandem DNA sites. EMBO J 1996; 15:5616 - 26; PMID: 8896455
- Murphy TL, Geissal ED, Farrar JD, Murphy KM. Role of the Stat4 N domain in receptor proximal tyrosine phosphorylation. Mol Cell Biol 2000; 20:7121 - 31; http://dx.doi.org/10.1128/MCB.20.19.7121-7131.2000; PMID: 10982828
- John S, Vinkemeier U, Soldaini E, Darnell JE Jr., Leonard WJ. The significance of tetramerization in promoter recruitment by Stat5. Mol Cell Biol 1999; 19:1910 - 8; PMID: 10022878
- Timofeeva OA, Tarasova NI. Alternative ways of modulating JAK-STAT pathway: Looking beyond phosphorylation. JAKSTAT 2012; 1:274 - 84; http://dx.doi.org/10.4161/jkst.22313; PMID: 24058784
- Lin JX, Li P, Liu D, Jin HT, He J, Ata Ur Rasheed M, Rochman Y, Wang L, Cui K, Liu C, et al. Critical Role of STAT5 transcription factor tetramerization for cytokine responses and normal immune function. Immunity 2012; 36:586 - 99; http://dx.doi.org/10.1016/j.immuni.2012.02.017; PMID: 22520852
- Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 2008; 133:1106 - 17; http://dx.doi.org/10.1016/j.cell.2008.04.043; PMID: 18555785
- Ji H, Jiang H, Ma W, Johnson DS, Myers RM, Wong WH. An integrated software system for analyzing ChIP-chip and ChIP-seq data. Nat Biotechnol 2008; 26:1293 - 300; http://dx.doi.org/10.1038/nbt.1505; PMID: 18978777
- Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 2010; 5:725 - 38; http://dx.doi.org/10.1038/nprot.2010.5; PMID: 20360767
- Zhao Y, Huang Y, Gong Z, Wang Y, Man J, Xiao Y. Automated and fast building of three-dimensional RNA structures. Sci Rep 2012; 2:734; http://dx.doi.org/10.1038/srep00734; PMID: 23071898
- Huang Y, Liu S, Guo D, Li L, Xiao Y. A novel protocol for three-dimensional structure prediction of RNA-protein complexes. Sci Rep 2013; 3:1887; http://dx.doi.org/10.1038/srep01887; PMID: 23712416
- Becker S, Groner B, Müller CW. Three-dimensional structure of the Stat3beta homodimer bound to DNA. Nature 1998; 394:145 - 51; http://dx.doi.org/10.1038/28101; PMID: 9671298
- Zhang L, Badgwell DB, Bevers JJ 3rd, Schlessinger K, Murray PJ, Levy DE, Watowich SS. IL-6 signaling via the STAT3/SOCS3 pathway: functional analysis of the conserved STAT3 N-domain. Mol Cell Biochem 2006; 288:179 - 89; http://dx.doi.org/10.1007/s11010-006-9137-3; PMID: 16718380
- Arnosti DN, Kulkarni MM. Transcriptional enhancers: Intelligent enhanceosomes or flexible billboards?. J Cell Biochem 2005; 94:890 - 8; http://dx.doi.org/10.1002/jcb.20352; PMID: 15696541
- Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-André V, Sigova AA, Hoke HA, Young RA. Super-enhancers in the control of cell identity and disease. Cell 2013; 155:934 - 47; http://dx.doi.org/10.1016/j.cell.2013.09.053; PMID: 24119843
- Hakim O, Sung M-H, Nakayamada S, Voss TC, Baek S, Hager GL. Spatial congregation of STAT binding directs selective nuclear architecture during T-cell functional differentiation. Genome Res 2013; 23:462 - 72; http://dx.doi.org/10.1101/gr.147652.112; PMID: 23212947
- Timofeeva OA, Chasovskikh S, Lonskaya I, Tarasova NI, Khavrutskii L, Tarasov SG, Zhang X, Korostyshevskiy VR, Cheema A, Zhang L, et al. Mechanisms of unphosphorylated STAT3 transcription factor binding to DNA. J Biol Chem 2012; 287:14192 - 200; http://dx.doi.org/10.1074/jbc.M111.323899; PMID: 22378781
- Chatterjee-Kishore M, Wright KL, Ting JP, Stark GR. How Stat1 mediates constitutive gene expression: a complex of unphosphorylated Stat1 and IRF1 supports transcription of the LMP2 gene. EMBO J 2000; 19:4111 - 22; http://dx.doi.org/10.1093/emboj/19.15.4111; PMID: 10921891
- Timofeeva OA, Tarasova NI, Zhang X, Chasovskikh S, Cheema AK, Wang H, Brown ML, Dritschilo A. STAT3 suppresses transcription of proapoptotic genes in cancer cells with the involvement of its N-terminal domain. Proc Natl Acad Sci U S A 2013; 110:1267 - 72; http://dx.doi.org/10.1073/pnas.1211805110; PMID: 23288901
- Shi S, Calhoun HC, Xia F, Li J, Le L, Li WX. JAK signaling globally counteracts heterochromatic gene silencing. Nat Genet 2006; 38:1071 - 6; http://dx.doi.org/10.1038/ng1860; PMID: 16892059
- Shi S, Larson K, Guo D, Lim SJ, Dutta P, Yan SJ, Li WX. Drosophila STAT is required for directly maintaining HP1 localization and heterochromatin stability. Nat Cell Biol 2008; 10:489 - 96; http://dx.doi.org/10.1038/ncb1713; PMID: 18344984
- Hu X, Dutta P, Tsurumi A, Li J, Wang J, Land H, Li WX. Unphosphorylated STAT5A stabilizes heterochromatin and suppresses tumor growth. Proc Natl Acad Sci U S A 2013; 110:10213 - 8; http://dx.doi.org/10.1073/pnas.1221243110; PMID: 23733954
- Timofeeva OA, Gaponenko V, Lockett SJ, Tarasov SG, Jiang S, Michejda CJ, Perantoni AO, Tarasova NI. Rationally designed inhibitors identify STAT3 N-domain as a promising anticancer drug target. ACS Chem Biol 2007; 2:799 - 809; http://dx.doi.org/10.1021/cb700186x; PMID: 18154267