Abstract
The concentrations of several pharmaceuticals and personal care products (PPCPs) were simulated in the Grand River watershed using the PhATE (Pharmaceutical Assessment and Transport Evaluation) model. PPCPs that were previously measured in the Grand River watershed were selected as the target compounds for this study. The application of the PhATE model was adapted to account for Canadian climatic conditions and its seasonal variability. In this regard, seasonal hydrological parameters (i.e., stream flow and velocity) were estimated based on historical data. Chemical loss parameters, including in-stream decay, human loss, and removal efficiency of treatment plants, were initially extracted from the literature and then calibrated to the site data. In general, the adapted PhATE model reasonably simulated pharmaceuticals with continuous use by humans such as ibuprofen, naproxen, carbamazepine, and gemfibrozil. Validation, which was performed only for ibuprofen, naproxen, and carbamazepine suggests that the calibrated model is able to reliably simulate concentrations. The model is the most accurate in validation when simulating carbamazepine which is the most persistent substance tested in this study, and as such, variations in its concentration primarily follow variation in stream flow.
Les concentrations de plusieurs produits pharmaceutiques et de soins personnels (PPSP) ont t simules dans le bassin de la rivire Grand l'aide du modle PhATE (Pharmaceutical Assessment and Transport Evaluation). Les PPSP qui avaient t mesurs antrieurement dans le bassin versant de la rivire Grand ont t slectionns en tant que composs cibles pour cette tude. L'application du modle PhATE a t adapte afin de tenir compte des conditions climatiques du Canada et de sa variabilit saisonnire. cet gard, les paramtres hydrologiques saisonniers (c.--d. le dbit et la vitesse dcoulement) ont t estims en fonction des donnes historiques. Les paramtres de perte chimique, y compris la dtrioration dans le cours d'eau, les pertes causes par les activits humaines et l'efficacit dpuration des stations de traitement, ont t extraits initialement de la documentation puis ont t talonns en fonction des donnes du site. En gnral, le modle PhATE adapt a permis de simuler dans une mesure raisonnable les produits pharmaceutiques avec utilisation humaine continue, comme l'ibuprofne, le naproxne, la carbamazpine et le gemfibrozil. La validation, qui n'a t excute que pour l'ibuprofne, le naproxne et la carbamazpine, porte croire que le modle talonn est capable de simuler les concentrations de manire fiable. L o le modle s'avre le plus prcis, au chapitre de la validation, c'est lorsqu'il simule la carbamazpine, substance la plus persistante teste dans la prsente tude. ce titre, les variations dans sa concentration suivent principalement la variation du dbit du cours d'eau.
Introduction
Pharmaceuticals and personal care products (PPCPs) are chemicals used extensively in society for treatment, prevention, and beautification. Humans ingest pharmaceuticals and related products almost daily, and a fraction of each dosage is excreted (i.e., as feces or urine) due to incomplete metabolism in the human body. This excreted fraction may ultimately be discharged to surface waters via waste water treatment plant discharges (Lissemore et al., Citation2006; Miao et al., Citation2002; Metcalfe et al., Citation2003; Kreisberg, Citation2007; Buser et al., Citation1999; Heberer, Citation2002; Ternes, Citation1998; Andreozzi et al., Citation2003; Bendz et al., Citation2005; Clara et al., Citation2005; Lindqvist et al., Citation2005; Nakada et al., Citation2007; Ying et al., Citation2009).
As a result of these discharges, detectable amounts of PPCPs have been reported in surface and ground waters and drinking water (Daughton and Ternes, Citation1999; Heberer, Citation2002; Buzby, Citation2005; Ternes et al., Citation1999; Carrara et al., Citation2008; Godfrey et al., Citation2007; Kolpin et al., Citation2002; Lissemore et al., Citation2006; Metcalfe et al., Citation2003; Miao et al., Citation2002). Canadian watersheds have been found to be impacted (Lissemore et al., Citation2006; Metcalfe et al., Citation2003; Miao et al., Citation2002; Brun et al., Citation2006; Hao et al., Citation2006; Lishman et al., Citation2006), although detected concentrations have generally been low, i.e., between nanograms and micrograms per liter (Lissemore et al., Citation2006; Ternes et al., Citation1999; Tixier et al., Citation2003). However, even at low concentrations, the continual discharge of these chemicals into the environment may have adverse health effects on aquatic biota, such as feminization of various species (Barcelo and Petrovic, Citation2007; Jobling et al., Citation1998). Therefore, the fate and transport of PPCPs in varying environments has emerged as an important research topic (Barcelo and Petrovic, Citation2007; Kreisberg, Citation2007).
The detection and identification of PPCP compounds in natural systems requires highly sensitive instruments, and these analyses consume considerable time and money. Therefore, there has been an increasing interest in the development of models capable of reliably predicting the fate of pharmaceuticals. For example, the GREAT-ER (Geo-referenced Regional Exposure Assessment Tool for European Rivers) and PhATE (Pharmaceutical Assessment and Transport Evaluation) models were developed to estimate the concentrations of chemicals in surface waters (Cunningham et al., Citation2010; Anderson et al., Citation2004; Feijtel et al., Citation1997; Cunningham, Citation2008; Lindberg et al., Citation2006; Robinson et al., Citation2007). The models can be employed to facilitate an assessment of the potential toxicity effect of pharmaceuticals on aquatic life (Cunningham, Citation2008; Hannah et al., Citation2009). Furthermore, they can help guide the design of cost-effective field sampling strategies by highlighting the compounds with higher potential risk (Cunningham, Citation2008; Hannah et al., Citation2009). While these models were developed in European and United States settings, the climate in Canada exhibits considerable seasonal variability, and the long cold season may affect the fate of pharmaceuticals and personal care products in the environment. Exploring whether the use of seasonal parameters could improve PhATE predictions was the main objective of this research. Therefore, in this study, the PhATE model was adapted to account for Canadian conditions by seasonally predicting concentrations of aquatic chemicals (i.e., spring, summer, fall, and winter). The Grand River watershed, located in Ontario, Canada, was used as a case study to evaluate the performance of the adapted PhATE model.
Methods
In this study, the PhATE model was employed in a manner that allowed various physical, chemical, and hydrological factors to vary with the seasons (i.e., spring, summer, fall, and winter). Initially, compound-specific values for model parameters, such as removal efficiency of treatment plants, in-stream loss, and human loss, were collected from the literature. After an assessment of the model performance, the parameters were calibrated using available data that were collected from the Grand River watershed. Observation data not used for calibration served as the basis for subsequent model validation, to verify whether the calibrated model could adequately predict a separate data set of PPCP concentrations.
PhATE Model
The PhATE (Pharmaceutical Assessment and Transport Evaluation) model was developed to simulate concentrations of active pharmaceutical ingredients in eleven watersheds across the United States (Anderson et al., Citation2004). The PhATE model is based upon mass balance equations that describe contaminant fate in each segment of the watershed. For each segment, mass enters either via inflow from upstream segments or from wastewater treatment plants as point sources along the segment. Contaminants leave the segment via first-order in-stream decay, flow diversions, or outflow to a downstream segment (Anderson et al., Citation2004). The mass of PPCPs entering from a given wastewater treatment plant is estimated as the product of the average annual human usage of the compounds per capita multiplied by the size of the population served by the wastewater treatment plants. This loading is reduced via two loss terms, namely: (1) removal by human metabolism; and (2) removal within the treatment plants (Anderson et al., Citation2004).
Grand River Watershed
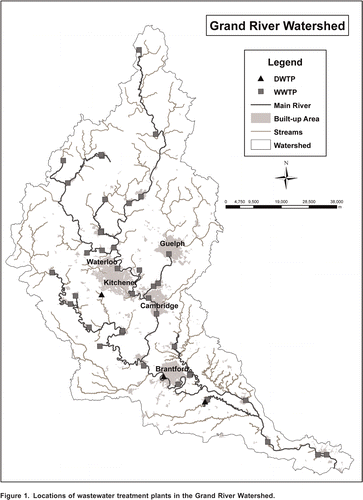
The Grand River watershed is located in southwestern Ontario and drains an area of about 6,800 km2 (Hao et al., Citation2006). The watershed receives treated effluent from a total of forty wastewater treatment plants which serve about 530,000 residents (). Twenty eight are municipal plants and, of these, fifteen provide advanced tertiary treatment, nine provide conventional secondary treatment, and four are lagoons (Koycheva, Citation2003).
Target Chemicals
The selection of compounds in this study was limited to those where seasonal measured data were available to allow a robust assessment of the model. These consisted of ibuprofen, naproxen, carbamazepine, gemfibrozil, sulfamethoxazole, trimethoprim, and bezafibrate. These compounds are the most frequently detected compounds in Canadian surface waters (Lissemore et al., Citation2006; Metcalfe et al., Citation2003; Brun et al., Citation2006; Hao et al., Citation2006; Lishman et al., Citation2006). The compounds span a range of persistence in wastewater treatment with naproxen and ibuprofen reported to have relatively high removals ranging from 89% to 99% (Metcalfe et al., Citation2003; Lishman et al., Citation2006), while carbamazepine has been reported to be recalcitrant (Metcalfe et al., Citation2003; Brun et al., 2006).
A thorough review of the literature was conducted to obtain model parameters for the target compounds (). From it can be seen that in almost all instances there is a wide range of removals reported for the target compounds. However, in general, when the removals are compared between compounds, the patterns in removal relative to each other are consistent across processes. The wide range of parameter values for compounds presents considerable challenges, and hence, necessitates the staged model calibration that is described in this paper. As will be subsequently described, the literature values in were used to conduct an initial round of simulations and to constrain the auto-calibration.
Table 1. Literature-derived ranges of parameter values for the selected compounds.
Hydrological Properties
The PhATE model employs information on average and low flows and the corresponding velocities for each stream segment. Seasonal hydrological parameters were estimated using flow values reported by the Grand River Conservation Authority (GRCA) and the Water Survey of Canada (WSC). In this study, the hydrological season was defined as three continuous months of relatively similar flows, and hence, they were categorized as follows: winter December, January, and February; spring March, April, and May; summer June, July, and August; and fall September, October, and November.
The seasonal mean and low flow for each stream segment was estimated using a relationship between flow and drainage area. Drainage area, precipitation, and temperature are the most important variables for estimating average annual flow (Vogel et al., Citation1999), and of these variables, drainage area is recognized as the most influential factor (Mohamoud and Pamar, Citation2006). In the Grand River watershed, drainage area is highly correlated with average and low seasonal flows and the regressions yielded R2 values ranging from 0.78 to 0.99 and 0.69 to 0.99, respectively.
The velocity in each segment was calculated using available flow data paired with relevant morphologic features of the stream segments reported in previous studies (Annable, Citation1996). The velocities were estimated using a logarithmic relationship between velocity and flow rate (Leopold and Maddock, Citation1953). Low-flow velocities for each stream segment were calculated using Equation
Equation 1
(United States Environmental Protection Agency (USEPA), Citation1997).
where Vlow is low flow velocity, Vavg is mean flow velocity, Qlow is low flow, and Qavg is mean flow.
Numerical Experiments
As previously described, an initial survey of the literature was performed in order to establish a reasonable range of expected values for these parameters. PhATE simulations were then conducted using average values for the parameters that were based on the literature ranges. In this part of the study, seasonal hydrologic parameters were incorporated into four input files, with one input file per season. Also included in each input file were corresponding seasonal estimates of pharmaceutical loading and the various loss parameters. The values of the parameters for PPCPs and endocrine-disrupting chemicals (EDCs) that were included in the individual files were categorized seasonally according to the reported time of the measurement, if it was available, and also involved the application of some expert judgment.
As will be subsequently demonstrated, the simulations with the literature-derived parameter values yielded unrealistic predictions for certain compounds (e.g., naproxen) when applied to the Grand River watershed. Hence, in the second stage of the study an automated calibration was conducted to obtain parameter values that best match observed behaviour in the Grand River watershed. Automated calibration was used to adjust uncertain model parameters in order to obtain the best possible correspondence between model outputs and historical observation data. Parameters including flow and velocity of stream segments and annual per capita pharmaceutical usage were derived from data that were readily available for the Grand River watershed and/or Canada, and hence, were not adjusted. Conversely, the removal efficiencies of lagoons, secondary and tertiary treatment plants, in-stream loss, and human loss were not known with certainty, and hence, were varied during the calibration exercise. A total of 94 data points were available for a location of the Grand River that is near Mannheim; these had been collected over a period that spanned from November 2006 to August 2008 (Halle, Citation2009). These concentrations of PPCPs served as the observational data set and were utilized for model calibration. Note that PhATE assumes steady-state, and thus, the model had to be re-run for each data point in the flow and concentration time-series.
In this project, the DDS (Dynamically Dimensioned Search) optimization algorithm (Tolson and Shoemaker, Citation2007) through the OSTRICH (Optimization Software Tool for Research In Computational Heuristics) interface (Matott, Citation2005) was employed. OSTRICH is specifically designed so that it can easily be linked with models that accept and generate text-based input/output files with no user interaction required. However, the PhATE model works only through a graphical user interface (GUI) and depends heavily on user-interaction via various button clicks and dialog windows. Furthermore, the PhATE model utilizes the Access Database file format for its input/output files. Therefore, the OSTRICH software was modified to support the Access Database file format in order to link OSTRICH with the PhATE model. Also, to programmatically manipulate the input of the PhATE model, AutoHotKey (http://www.autohotkey.com, an open-source utility for windows that can simulate keystrokes and mouse clicks) was used to allow OSTRICH to run the PhATE model without user intervention.
Calibration was conducted using the average parameter values as the initial solution and was constrained by the parameter values reported in the literature. Calibration required definition of an objective function, and in this study minimizing the conventional weighted sum of squared deviations of simulated concentration values from measured data was utilized (Hill and Tiedeman, Citation2007). Equal importance (weights) was assumed for all observations while calibrating for all compounds except for bezafibrate and sulfamethoxazole. For calibration of these two compounds, the measured concentrations above the detection limit received a greater weight (i.e., a weight twice as large as the weight of observations below the detection limit).
Two hundred function evaluations were employed in the auto-calibration process. Each function evaluation consisted of n PhATE model runs, where n is the number of available observations in each season. For example, for the ibuprofen calibration in winter, each function evaluation consisted of 20 PhATE model runs, one for each of the input files. For this study, a computational budget of 200 function evaluations (or about 4.5 hours) was assigned to each calibration trial, with multiple trials required for calibrating seasonal behavior.
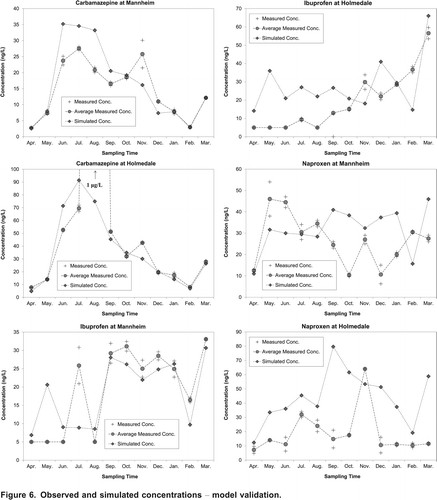
Following calibration, a validation exercise assessed the ability of the calibrated model to adequately predict PPCP concentrations. A limited number of seasonal data was available for validation, and hence, the validation was performed for three compounds, ibuprofen, naproxen, and carbamazepine. The validation data included the concentrations that were collected at two different locations in the Grand River watershed, near Mannheim and Holmedale (Kormos, Citation2007). Two samples had been collected at each location monthly from April 2005 to March 2006 (Kormos, Citation2007). These pharmaceuticals were simulated using the seasonal calibrated parameters and actual flows at the time of sampling. The seasonal simulated concentrations were compared to the seasonal measured data and were deemed to have a favorable match if the predicted values were within a factor of ten of the measured data (Anderson et al., Citation2004). The value of the sum of squared deviations of simulated concentration values from measured data was also employed to assess the goodness of fit of the model during validation.
Results
Simulations with Literature-Derived Parameter Values
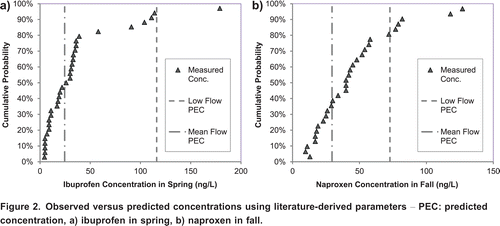
The cumulative distribution of the measured concentrations was used to assess the ability of the model to reliably predict the range of concentrations that were observed for each season. As an example, shows the predicted concentrations (PECs) of ibuprofen in the spring and naproxen in the fall along with the cumulative distribution function of the corresponding measured concentration values. The results showed that for ibuprofen and naproxen about 50% of the observed values in each season fall within the range of the simulated concentrations associated with average seasonal mean flow and average seasonal low flow values. However, in the fall, only 2030% of ibuprofen and naproxen measured concentrations fall within the corresponding simulated concentration ranges. The simulated concentrations of carbamazepine show good agreement with the measured data. Only one measured data point in the spring and one in the summer exceed the low flow-based simulated concentration.
Auto-Calibration Results
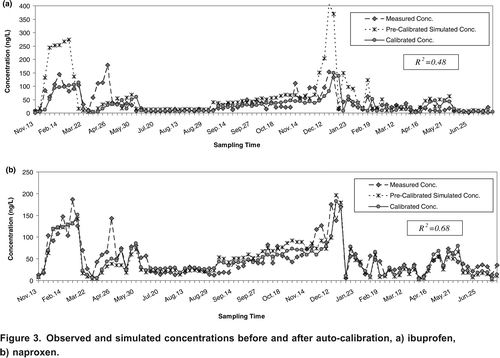
The five PhATE model parameters (i.e., removal efficiencies in lagoon, secondary and tertiary treatment plants, in-stream decay, and human loss) were seasonally calibrated to obtain model results that best match the observed concentrations in the Grand River watershed. The PhATE model performance before and after auto-calibration was compared for each compound. As examples of this comparison, the simulated concentrations of ibuprofen and naproxen are shown versus the corresponding measured data in . From these graphs it can be observed that the PhATE model is capable of predicting the concentrations of the PPCPs when actual flows are used, and calibration results in a significantly better estimation (compared to pre-calibration) of the concentrations in the Grand River watershed. The PhATE model is able to better simulate ibuprofen, naproxen, carbamazepine, and gemfibrozilas compared to other compounds. The estimated R2 values for these drugs ranges from 0.48 for ibuprofen to 0.69 for gemfibrozil.
Figure 3. Observed and simulated concentrations before and after auto-calibration, a) ibuprofen, b) naproxen.
shows the calibrated parameter values, which were referred to as the most probable seasonal parameters of the selected compounds for the case study. As shown in , for the majority of pharmaceuticals, the removal efficiency of the tertiary treatment plants has the highest removal rates among the removal efficiencies of the different treatment plants. However, for ibuprofen and naproxen, lagoons are found to be the most efficient treatment plants. The efficiency of the treatment plants in removing naproxen, carbamazepine and bezafibrate varies seasonally. For example, naproxen has the highest removal efficiency in the treatment plants in the summer season. However, there is no significant seasonal variability in the removal efficiencies of the treatment plants for ibuprofen and trimethoprim. also shows that the calibrated human loss values for each compound varies modestly between seasons. It might be expected that human loss would be essentially constant for all seasons. Hence, the variation is attributed to uncertainties in modelling and calibration and/or seasonality in loading. As loading was fixed in the calibration step for each season, seasonality in loading would be reflected in variability in the human loss parameter.
Table 2. Calibrated parameter values.
For some cases, the calibrated parameter values seem to be unreasonable; for instance, high variability in in-stream decay value during a year (e.g., two or three orders of magnitude), a large difference between removal efficiencies of a treatment plant between seasons, and lower removal rates in summer than in winter. Potential explanations for these patterns include: (1) the calibration was performed only at one sample location which was mostly affected by secondary treatment plant removal; therefore, in-stream decay, lagoon, and tertiary treatment plant removal were not sufficiently influential parameters for the calibration; (2) a high percent of observed concentrations for some of the compounds were reported below detection limit (e.g., bezafibrate); and (3) correlation between parameters in the calibration exercise.
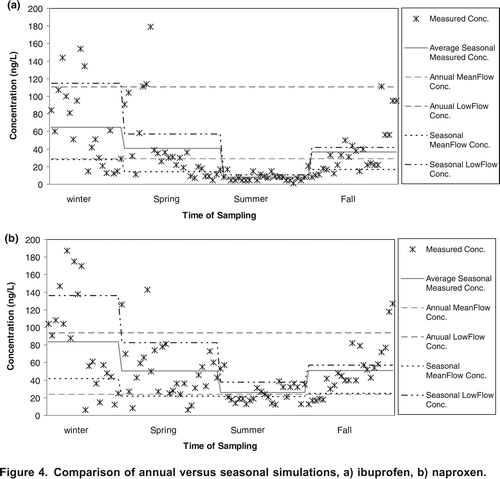
In order to assess the significance of applying seasonality to the model parameters in this study, two scenarios, annual and seasonal, were considered. In the annual scenario, all the hydrological and chemical parameters were assumed to be constant over the year and annual mean flows were employed. In the seasonal scenarios the hydrological and chemical parameters were assumed to vary with season. The average annual and seasonal simulated concentrations of ibuprofen and naproxen are plotted in along with the measured concentrations. The comparison is based on the average simulated and measured concentrations for each season. As shown in , incorporating seasonality of parameters improves the simulation of ibuprofen and naproxenwhen compared to the observed data.
The improvement in seasonally predicting pharmaceutical concentrations was further assessed by comparing the percentage of the field measured concentrations that were between the simulated mean and low flow concentrations for each season. The results indicate that simulation of ibuprofen and naproxen improves after seasonal calibration in most of the seasons, as a larger percentage of the measured data is between the simulated seasonal mean and low flow concentrations. In the winter, the percent of measured data between seasonal mean and low flow concentrations increases from 55 to 60% for ibuprofen and from 4 to 50% for naproxen. While the use of seasonal parameters for naproxen improves simulations in the winter and the summer, the number of measured concentrations within the two predicted concentrations in the spring and the fall decreases. A higher number of measured concentrations of carbamazepine fall between the simulated concentrations when annual parameters are applied.
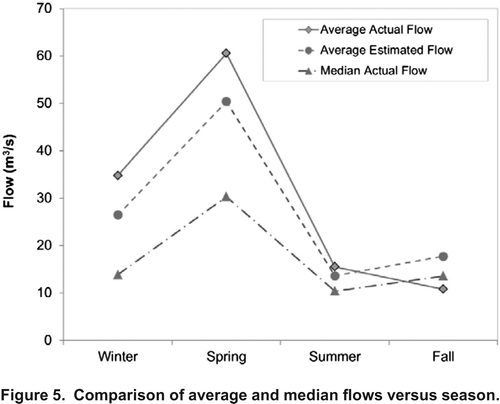
Beside seasonality of parameters, other factors may improve the simulation results. There are good fits between measured and calibrated concentrations when the flow rates that were recorded at the time of sample collection are used, although the average simulated concentrations are less than the average measured concentrations for all of the seasons and compounds. This result suggests that the average flow might not be the best strategy in estimating the average concentrations, and as lower flow values would typically result in higher concentrations, other types of central tendency measures, like the median flows, which are typically less than average streamflows, might be preferred. compares the average and median flow values at the time of sampling with the average estimated flows, and shows the median flow rates were considerably lower than the average flow. As the estimated concentrations in all cases are less than the average measured concentrations, accounting for the median flow instead of mean flow is expected to increase the simulated concentration values and improve the seasonal simulations.
Validation Results
In the validation phase, ibuprofen, naproxen, and carbamazepine were simulated using the seasonally calibrated parameters and actual flows at the time of sampling. shows the simulated concentrations of ibuprofen, naproxen, and carbamazepine along with the corresponding measured concentration values. For carbamazepine, as shown in , there is a good fit between the simulated and the measured concentrations at both sampled locations, with the exception of the August sample event at Holmedale, when the measured concentration was extremely high (likely an outlier), about 1 g/L. For ibuprofen, the simulated concentrations and the measured concentrations at Mannheim have a good fit, while the simulations at Holmedale do not match the measured data sufficiently well. The better model performance for Mannheim is probably due to the fact that the calibration was also performed on this segment, while Holmedale was not considered in calibration. Note that this behavior (different accuracies in the Grand River near Mannheim and Holmedale) is not observed for carbamazepine, possibly because this compound is relatively persistent in the environment. Therefore, the variation in carbamazepine concentration is generally governed by the variation in the flow rate. The validation results for naproxen are not as good as the validation results for ibuprofen and carbamazepine at both locations. The sum of squared deviations (SSD) of simulated concentration of naproxen from measured data at Mannheim was 2,521 which is one order of magnitude larger that ibuprofen SSD (485) and carbamazepine SSD (332).
Although the accuracy in validation might not seem very high, previous researchers have suggested that simulations of this class of chemicals are reasonable if the predicted data are within a factor of ten of the measured data (Anderson et al., Citation2004). While the degree of model accuracy that is deemed to be acceptable will depend upon the intended use of the simulations, the validation results in this study indicate relatively good fits between observed data and corresponding model output.
Conclusions
This study employed the PhATE model to predict transport of frequently detected pharmaceuticals, personal care products, and endocrine disruptors in the Grand River watershed. When the application of the PhATE model was adapted to reflect seasonal conditions it was found to be capable of accurately simulating pharmaceutical concentrations in this watershed. In the pre-calibration phase (i.e., using previously published parameter values), the model tended to over predict concentrations.
After calibration, the PhATE model accurately predicted pharmaceutical concentrations in the Grand River. The use of accurate values for stream flow and velocity was found to be important for estimation of PPCPs in the surface water. In the validation phase, the performance of the calibrated PhATE model was tested with a set of data that was not used in calibration at two sampling sites. The validation results showed relatively good fit between the measured data and corresponding model output, and the deviations of the simulated concentrations from the measured data were consistently less than a factor of ten.
Acknowledgements
The authors acknowledge Webber Chan who modified the OSTRICH software to support Access Database and the use of AutoHotkey.exe to run the PhATE model automatically.
References
- Anderson , P. D. , DAco , V. J. , Shanahan , P. , Chapra , S. C. , Buzby , M. E. , Cunningham , V. L. and Duplessie , B. M. 2004 . Screening analysis of human pharmaceutical compounds in US surface waters . Environmental Science & Technology , 38 ( 3 ) : 838 – 849 .
- Andreozzi , R. , Raffaele , M. and Nicklas , P. 2003 . Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment . Chemosphere , 50 ( 10 ) : 1319 – 1330 .
- Annable, W. K. 1996. Database of morphological characteristics of watercourses in southern Ontario. Toronto: Ontario Ministry of Natural Resources, 188 pp.
- Barcelo , D. and Petrovic , M. 2007 . Pharmaceuticals and personal care products (PPCPs) in the environment . Analytical and Bioanalytical Chemistry , 387 ( 4 ) : 1141 – 1142 .
- Batt , A. L. , Kim , S. and Aga , D. S. 2006 . Enhanced biodegradation of iopromide and trimethoprim in nitrifying activated sludge . Environmental Science & Technology , 40 ( 23 ) : 7367 – 7373 .
- Bendz , D. , Paxeus , N. A. , Ginn , T. R. and Loge , F. J. 2005 . Occurrence and fate of pharmaceutically active compounds in the environment, a case study: Hoje River in Sweden . Journal of Hazardous Materials , 122 ( 3 ) : 195 – 204 .
- Brown , K. D. , Kulis , J. , Thomson , B. , Chapman , T. H. and Mawhinney , D. B. 2006 . Occurrence of antibiotics in hospital, residential, and dairy, effluent, municipal wastewater, and the Rio Grande in New Mexico . Science of the Total Environment , 366 ( 23 ) : 772 – 783 .
- Brun , G. L. , Bernier , M. , Losier , R. , Doe , K. , Jackman , P. and Lee , H. B. 2006 . Pharmaceutically active compounds in Atlantic Canadian sewage treatment plant effluents and receiving waters, and potential for environmental effects as measured by acute and chronic aquatic toxicity . Environmental Toxicology and Chemistry , 25 ( 8 ) : 2163 – 2176 .
- Buser , H. R. , Poiger , T. and Muller , M. D. 1999 . Occurrence and environmental behavior of the chiral pharmaceutical drug ibuprofen in surface waters and in wastewater . Environmental Science & Technology , 33 ( 15 ) : 2529 – 2535 .
- Buzby, M. E. 2005. Pharmaceuticals in the environment. Paper presented at EPA Region 2 Science Day-Pharmaceuticals & Personal Care Products (PPCP) Workshop, October 2526, 2005, New York City, New York.
- Carrara , C. , Ptacek , C. J. , Robertson , W. D. , Blowes , D. W. , Moncur , M. C. , Sverko , E. and Backus , S. 2008 . Fate of pharmaceutical and trace organic compounds in three septic system plumes, Ontario, Canada . Environmental Science & Technology , 42 ( 8 ) : 2805 – 2811 .
- Castiglioni , S. , Bagnati , R. , Fanelli , R. , Pomati , F. , Calamari , D. and Zuccato , E. 2006 . Removal of pharmaceuticals in sewage treatment plants in Italy . Environmental Science & Technology , 40 ( 1 ) : 357 – 363 .
- Choi , K. , Kim , Y. , Park , J. , Park , C. K. , Kim , M. , Kim , H. S. and Kim , P. 2008 . Seasonal variations of several pharmaceutical residues in surface water and sewage treatment plants of Han River, Korea . Science of the Total Environment , 405 ( 13 ) : 120 – 128 .
- Clara , M. , Strenn , B. , Gans , O. , Martinez , E. , Kreuzinger , N. and Kroiss , H. 2005 . Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants . Water Research , 39 ( 19 ) : 4797 – 4807 .
- Conkle , J. L. , White , J. R. and Metcalfe , C. D. 2008 . Reduction of pharmaceutically active compounds by a lagoon wetland wastewater treatment system in southeast Louisiana . Chemosphere , 73 ( 11 ) : 1741 – 1748 .
- Cunningham , V. L. , Perino , C. , DAco , V. J. , Hartmann , A. and Bechter , R. 2010 . Human health risk assessment of carbamazepine in surface waters of North America and Europe . Regulatory Toxicology and Pharmacology , 56 ( 3 ) : 343 – 351 .
- Cunningham, V. L. 2008. Environmental exposure modeling: Application of PhATE and GREAT-ER to human pharmaceuticals in the environment. Chap. 10 in Pharmaceuticals in the environment-sources, fate, effects and risks, ed. K. Kmmerer. 133146. Berlin, Germany: Springer-Verlag.
- Daughton , C. G. and Ternes , T. A. 1999 . Pharmaceuticals and personal care products in the environment: Agents of subtle change? . Environmental Health Perspectives , 107 : 907 – 938 .
- Feijtel , T. , Boeije , G. , Matthies , M. , Young , A. , Morris , G. , Gandolfi , C. and Hansen , B. 1997 . Development of a geography-referenced regional exposure assessment tool for uropean Rivers-GREAT-ER Contribution to GREAT-ER #1 . Chemosphere , 34 ( 11 ) : 2351 – 2373 .
- Fono , L. J. , Kolodziej , E. P. and Sedlak , D. L. 2006 . Attenuation of wastewater-derived contaminants in an effluent-dominated river . Environmental Science & Technology , 40 ( 23 ) : 7257 – 7262 .
- Gobel , A. , Thomsen , A. , McArdell , C. S. , Joss , A. and Giger , W. 2005 . Occurrence and sorption behavior of sulfonamides, macrolides, and trimethoprim in activated sludge treatment . Environmental Science & Technology , 39 ( 11 ) : 3981 – 3989 .
- Gobel , A. , McArdell , C. S. , Joss , A. , Siegrist , H. and Giger , W. 2007 . Fate of sulfonamides, macrolides, and trimethoprim in different wastewater treatment technologies . Science of the Total Environment , 372 ( 23 ) : 361 – 371 .
- Godfrey , E. , Woessner , W. W. and Benotti , M. J. 2007 . Pharmaceuticals in on-site sewage effluent and ground water, Western Montana . Ground Water , 45 ( 3 ) : 263 – 271 .
- Gulkowska , A. , Leung , H. W. , So , M. K. , Taniyasu , S. , Yamashita , N. , Yeunq , L. W. Y. , Richardson , B. J. , Lei , A. P. , Giesy , J. P. and Lam , P. K. S. 2008 . Removal of antibiotics from wastewater by sewage treatment facilities in Hong Kong and Shenzhen, China . Water Research , 42 ( 12 ) : 395 – 403 .
- Halle, C. 2009. Biofiltration in drinking water treatment: Reduction of membrane fouling and biodegradation of organic trace contaminants. PhD Thesis, Civil and Environmental Engineering, University of Waterloo, Waterloo, Ontario, Canada, 329 pp.
- Hannah , R. , DAco , V. J. , Anderson , P. D. , Buzby , M. E. , Caldwell , D. J. , Cunningham , V. L. and Ericson , J. F. 2009 . Exposure assessment of 17 alpha-ethinylestradiol in surface waters of the United States and Europe . Environmental Toxicology and Chemistry , 28 ( 12 ) : 2725 – 2732 .
- Hao , C. Y. , Lissemore , L. , Nguyen , B. , Kleywegt , S. , Yang , P. and Solomon , K. 2006 . Determination of pharmaceuticals in environmental waters by liquid chromatography/electrospray ionization/tandem mass spectrometry . Analytical and Bioanalytical Chemistry , 384 ( 2 ) : 505 – 513 .
- Heberer , T. 2002 . Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a Review of recent research data . Toxicology Letters , 131 ( 12 ) : 5 – 17 .
- Hill, M. C., and C. R. Tiedeman. 2007. Effective groundwater model calibration: With analysis of data, sensitivities, predictions, and uncertainty. Hoboken, NJ: Wiley and Sons, 455 pp.
- Jobling , S. , Nolan , M. , Tyler , C. R. , Brighty , G. and Sumpter , J. P. 1998 . Widespread sexual disruption in wild fish . Environmental Science & Technology , 32 ( 17 ) : 2498 – 2506 .
- Jones , O. A. H. , Voulvoulis , N. and Lester , J. N. 2007 . The occurrence and removal of selected pharmaceutical compounds in a sewage treatment works utilising activated sludge treatment . Environmental Pollution , 145 ( 3 ) : 738 – 744 .
- Joss , A. 2005 . Removal of pharmaceuticals and fragrances in biological wastewater treatment . Water Research , 39 ( 14 ) : 3139 – 3152 .
- Karthikeyan , K. G. and Meyer , M. T. 2006 . Occurrence of antibiotics in wastewater treatment facilities in Wisconsin, USA . Science of the Total Environment , 361 ( 13 ) : 196 – 207 .
- Kasprzyk-Hordern , B. , Dinsdale , R. M. and Guwy , A. J. 2010 . The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters . Water Research , 43 ( 2 ) : 363 – 380 .
- Kolpin , D. W. , Furlong , E. T. , Meyer , M. T. , Thurman , E. M. , Zaugg , S. D. , Barber , L. B. and Buxton , H. T. 2002 . Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 19992000: A national reconnaissance . Environmental Science & Technology , 36 ( 6 ) : 1202 – 1211 .
- Kormos, J. L. 2007. Occurrence and seasonal variability of selected pharmaceuticals in southern Ontario drinking water supplies. Master's Thesis, Biology, University of Waterloo, Waterloo, Ontario, Canada, 162 pp.
- Koycheva, J. 2003. Drinking water quality in the Grand River watershed. Briefs on drinking water. Toronto: Canadian Institute for Environmental Law and Policy (CIELAP), 27 pp.
- Kreisberg , J. 2007 . Greener pharmacy: proper medicine disposal protects the environment . Integrative Medicine , 6 ( 4 ) : 50 – 52 .
- Kunkel , U. and Radke , M. 2008 . Biodegradation of acidic pharmaceuticals in bed sediments: Insight from a laboratory experiment . Environmental Science & Technology , 42 ( 19 ) : 7273 – 7279 .
- Lam , M. W. and Mabury , S. A. 2005 . Photodegradation of the pharmaceuticals atorvastatin, carbamazepine, levofloxacin, and sulfamethoxazole in natural waters . Aquatic Sciences , 67 ( 2 ) : 177 – 188 .
- Lee , R. B. , Sarafin , K. , Peart , T. E. and Svoboda , M. L. 2003 . Acidic pharmaceuticals in sewage-methodology, stability test, occurrence, and removal from Ontario samples . Water Quality Research Journal of Canada , 38 ( 4 ) : 667 – 682 .
- Leifer, A. 1988. The kinetics of environmental aquatic photochemistry: Theory and practice. Washington D.C: American Chemical Society, 261 pp.
- Leopold, L. B., and T. J. Maddock. 1953. The hydraulic geometry of stream channels and some physiographic implications. Washington, D.C: United States Geological Survey, 57 pp.
- Lienert , J. , Gudel , K. and Escher , B. I. 2007 . Screening method for ecotoxicological hazard assessment of 42 pharmaceuticals considering human metabolism and excretory routes . Environmental Science & Technology , 41 ( 12 ) : 4471 – 4478 .
- Lin , A. Y. C. , Yu , T. H. and Lateef , S. K. 2009 . Removal of pharmaceuticals in secondary wastewater treatment processes in Taiwan . Journal of Hazardous Materials , 167 ( 13 ) : 1163 – 1169 .
- Lindberg , R. H. , Olofsson , U. , Rendahl , P. , Johansson , M. I. , Tysklind , M. and Andersson , B. A. V. 2006 . Behavior of fluoroquinolones and trimethoprim during mechanical, chemical, and active sludge treatment of sewage water and digestion of sludge . Environmental Science & Technology , 40 ( 3 ) : 1042 – 1048 .
- Lindqvist , N. , Tuhkanen , T. and Kronberg , L. 2005 . Occurrence of acidic pharmaceuticals in raw and treated sewages and in receiving waters . Water Research , 39 ( 11 ) : 2219 – 2228 .
- Lishman , L. , Smyth , S. A. , Sarafin , K. , Kleywegt , S. , Toito , J. , Peart , T. , Lee , B. , Servos , M. , Beland , M. and Seto , P. 2006 . Occurrence and reductions of pharmaceuticals and personal care products and estrogens by municipal wastewater treatment plants in Ontario, Canada . Science of the Total Environment , 367 ( 23 ) : 544 – 558 .
- Lissemore , L. , Hao , C. Y. , Yang , P. , Sibley , P. K. , Mabury , S. and Solomon , K. R. 2006 . An exposure assessment for selected pharmaceuticals within a watershed in southern Ontario . Chemosphere , 64 ( 5 ) : 717 – 729 .
- Matamoros , V. , Duhec , A. , Albaiges , J. and Bayona , J. M. 2009 . Photodegradation of carbamazepine, ibuprofen, ketoprofen and 17 alpha-ethinylestradiol in fresh and seawater . Water Air and Soil Pollution , 196 ( 14 ) : 161 – 168 .
- Matott, L. S. 2005. Ostrich: An optimization software tool, documentation and user's guide, Version 1.6. Buffalo, NY: University at Buffalo, Department of Civil, Structural, and Environmental Engineering, 114 pp.
- Metcalfe , C. D. , Koenig , B. G. , Bennie , D. T. , Servos , M. , Ternes , T. A. and Hirsch , R. 2003 . Occurrence of neutral and acidic drugs in the effluents of Canadian sewage treatment plants . Environmental Toxicology and Chemistry , 22 ( 12 ) : 2872 – 2880 .
- Miao , X. S. , Koenig , B. G. and Metcalfe , C. D. 2002 . Analysis of acidic drugs in the effluents of sewage treatment plants using liquid chromatography-electrospray ionization tandem mass spectrometry . Journal of Chromatography A , 952 ( 12 ) : 139 – 147 .
- Mohamoud , Y. M. and Parmar , R. S. 2006 . Estimating streamflow and associated hydraulic geometry, the mid-atlantic region, USA . Journal of the American Water Resources Association , 42 ( 3 ) : 755 – 768 .
- National Library of Medicine NLM. 2010. DailyMed current medication information. http://dailymed.nlm.nih.gov/dailymed/about.cfm (accessed October 2010).http://dailymed.nlm.nih.gov/dailymed/about.cfm
- Nakada , N. , Shinohara , H. , Murata , A. , Kiri , K. , Managaki , S. , Sato , N. and Takada , H. 2007 . Removal of selected pharmaceuticals and personal care products PPCPs and endocrine-disrupting chemicals (EDCs) during sand filtration and ozonation at a municipal sewage treatment plant . Water Research , 41 : 4373 – 4382 .
- Packer , J. L. , Werner , J. J. , Latch , D. E. , McNeill , K. and Arnold , W. A. 2003 . Photochemical fate of pharmaceuticals in the environment: Naproxen, diclofenac, clofibric acid, and ibuprofen . Aquatic Sciences , 65 ( 4 ) : 342 – 351 .
- Paxeus , N. 2004 . Removal of selected non-steroidal anti-inflammatory drugs (NSAIDs), gemfibrozil, carbamazepine, beta-blockers, trimethoprim and triclosan in conventional wastewater treatment plants in five EU countries and their discharge to the aquatic environment . Water Science and Technology , 50 ( 5 ) : 253 – 260 .
- Radke , M. , Ulrich , H. , Wurm , C. and Kunkel , U. 2010 . Dynamics and attenuation of acidic pharmaceuticals along a river stretch . Environmental Science & Technology , 44 ( 8 ) : 2968 – 2974 .
- Roberts , P. H. and Thomas , K. V. 2006 . The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment . Science of the Total Environment , 356 ( 13 ) : 143 – 153 .
- Robinson , P. F. , Liu , Q. T. , Riddle , A. M. and Murray-Smith , R. 2007 . Modeling the impact of direct phototransformation on predicted environmental concentrations PECs of propranolol hydrochloride in UK and US rivers . Chemosphere , 66 ( 4 ) : 757 – 766 .
- Runkel , R. , Forchielli , E. , Boost , G. , Chaplin , M. , Hill , R. and Sevelius , H. 1973 . Naproxen-metabolism, excretion and comparative pharmacokinetics . Scandinavian Journal of Rheumatology-Supplement , 2 : 24 – 36 .
- Santos , J. L. , Aparicio , I. and Alonso , E. 2007 . Occurrence and risk assessment of pharmaceutically active compounds in wastewater treatment plants-a case study: Seville City (Spain) . Environment International , 33 ( 4 ) : 596 – 601 .
- Smith , T. C. 1976 . Toleration and bioavailability of gemfibrozil in healthy men . Proceedings of the Royal Society of Medicine-London , 69 : 24 – 27 .
- Ternes , T. A. 1998 . Occurrence of drugs in German sewage treatment plants and rivers . Water Research , 32 ( 11 ) : 3245 – 3260 .
- Ternes , T. A. , Stumpf , M. , Mueller , J. , Haberer , K. , Wilken , R. D. and Servos , M. 1999 . Behavior and occurrence of estrogens in municipal sewage treatment plants-(i) investigations in Germany, Canada and Brazil . Science of the Total Environment , 225 ( 12 ) : 81 – 90 .
- Tixier , C. , Singer , H. P. , Oellers , S. and Muller , S. R. 2003 . Occurrence and fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofen, and naproxen in surface waters . Environmental Science & Technology , 37 ( 6 ) : 1061 – 1068 .
- Tolson , B. A. and Shoemaker , C. A. 2007 . Dynamically dimensioned search algorithm for computationally efficient watershed model calibration . Water Resources Research , 43 : W01413
- Trovo , A. G. , Nogueira , R. F. P. , Aguera , A. , Sirtori , C. and Fernandez-Alba , A. R. 2009 . Photodegradation of sulfamethoxazole in various aqueous media: Persistence, toxicity and photoproducts assessment . Chemosphere , 77 ( 10 ) : 1292 – 1298 .
- United States Environmental Protection Agency (USEPA). 1997. Streams and rivers, part 1: Biochemical oxygen demand/dissolved oxygen and nutrients/eutrophication. Technical guidance manual for developing total maximum daily loads, Book 2. EPA-823-B-97-002. Washington, D.C: United States Environmental Protection Agency (USEPA), 258 pp.
- Vogel , R. M. , Wilson , I. and Daly , C. 1999 . Regional regression models of annual streamflow for the United States . Journal of Irrigation and Drainage Engineering-ASCE , 125 ( 3 ) : 148 – 157 .
- Watkinson , A. J. , Murby , E. J. and Costanzo , S. D. 2007 . Removal of antibiotics in conventional and advanced wastewater treatment: Implications for environmental discharge and wastewater recycling . Water Research , 41 : 4164 – 4176 .
- Werner , J. J. , Boreen , A. L. , Edhlund , B. , Wammer , K. H. , Matzen , E. , McNeill , K. and Arnold , W. A. 2005 . Photochemical transformation of antibiotics in Minnesota waters . CURA Reporter , 35 ( 2 ) : 1 – 5 .
- Yamamoto , H. , Nakamura , Y. , Moriguchi , S. , Honda , Y. , Tamura , I. , Hirata , Y. , Hayashi , A. and Sekizawa , J. 2009 . Persistence and partitioning of eight selected pharmaceuticals in the aquatic environment: Laboratory photolysis, biodegradation, and sorption experiments . Water Research , 43 ( 2 ) : 351 – 362 .
- Ying , G. G. , Kookana , R. S. and Kolpin , D. W. 2009 . Occurrence and removal of pharmaceutically active compounds in sewage treatment plants with different technologies . Journal of Environmental Monitoring , 11 ( 8 ) : 1498 – 1505 .
- Yu , J. T. , Bouwer , E. J. and Coelhan , M. 2006 . Occurrence and biodegradability studies of selected pharmaceuticals and personal care products in sewage effluent . Agricultural Water Management , 86 ( 12 ) : 72 – 80 .
- Yu , T. H. , Lin , A. Y. C. , Lateef , S. K. , Lin , C. F. and Yang , P. Y. 2009 . Removal of antibiotics and non-steroidal anti-inflammatory drugs by extended sludge age biological process . Chemosphere , 77 ( 2 ) : 175 – 181 .
- Zhang , Y. J. , Geissen , S. U. and Gal , C. 2008 . Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies . Chemosphere , 73 ( 8 ) : 1151 – 1161 .