Abstract
Arbuscular mycorrhizas support nutrient uptake from the soil. Here we demonstrated Iron-59 (59Fe) uptake in maize (Zea mays L.) through the mycorrhizal roots. Arbuscular mycorrhizal colonization did not strongly induce Strategy II-related genes, but the putative iron transporter genes, OPT8a and OPT8b, were induced by more than 50-fold. Our data provide a previously undescribed gene expression mode related to the iron uptake system of Strategy II plants.
INTRODUCTION
Iron (Fe) is an essential nutrient for plants, but its bioavailability is often severely limited under aerobic soil conditions at neutral or alkaline pH (Marschner Citation2012). To overcome this difficulty, gramineous species have evolved the Strategy II iron uptake system, in which plants synthesize mugineic acids (MAs) through three sequential enzymatic reactions mediated by nicotianamine synthase (NAS), nicotianamine aminotransferase (NAAT) and deoxymugineic acid synthase (DMAS), generating 2’-deoxymugineic acid (DMA), the precursor of MAs, secrete them to the rhizosphere and chelate Fe(III) at the root periphery to form an Fe(III)-MAs complex. The complex is then taken up into roots via YELLOW STRIPE 1-like (YSL) transporters. Disruption of YS1 in maize (Zea mays L.) shows the phenotype of yellow-striped leaves reflecting Fe deficiency. These mechanisms have been recently reviewed (Curie et al. Citation2008; Conte and Walker Citation2011; Kobayashi and Nishizawa Citation2012).
A number of plant species form a symbiotic association with arbuscular mycorrhizal (AM) fungi in roots and efficiently absorb nutrients via fungi (Smith and Read Citation2008). Several studies have investigated the effect of mycorrhizal colonization on Fe uptake. The majority of the studies, but not all, have provided evidence that fungal inoculation can beneficially affect plant Fe nutrition (Clark and Zeto Citation2000). However, the molecular mechanism related to Fe uptake in mycorrhizal roots remains mostly unclear.
In this work, evidence of Fe uptake through AM fungi in roots of maize is presented. We also investigated the transcript levels of putative genes responsible for Fe acquisition system in mycorrhizal roots and found inductions of oligopeptide transporter (OPT) genes, OPT8a and OPT8b, in mycorrhizal roots. Although the substrate specificity of the OPT family is broad and the form of Fe delivered in mycorrhizal roots is unclear, some OPTs have Fe transport activity in the yeast heterologous expression system (Lubkowitz Citation2011). OPT8a and OPT8b may have a role in Fe delivery in maize mycorrhizal roots.
Figure 1 Iron-59 accumulation in the shoots of inoculated or non-inoculated Zea mays L. in two-compartment cultivation system. Four maize plants for each treatment (inoculated, Myc+; non-inoculated, Myc–) were grown in two-compartment cultivation system. The shoots were harvested and analyzed at 44 days post planting (dpp). Iron-59 (III) chloride was injected into hyphal compartment at 14 dpp. Data are given as means ± standard deviation (SD) of four biological replicates. N.D., not detected.
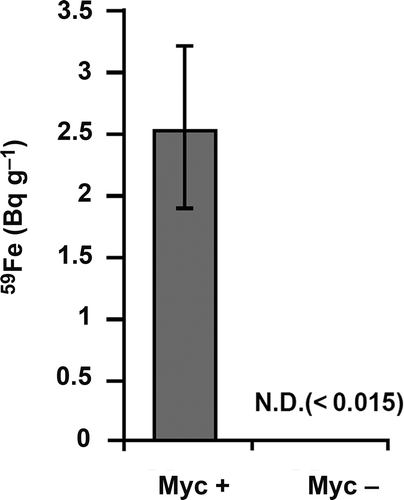
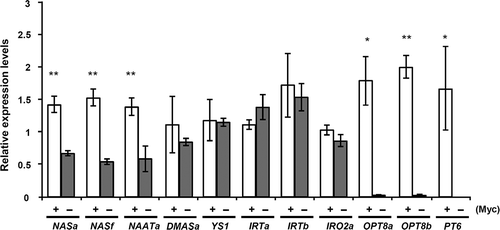
Figure 2 The expression analysis of genes related to Iron uptake in mycorrhizal or non-mycorrhizal roots of maize. Real-time RT-PCR-based expression analysis of maize genes related to Iron uptake system in the mycorrhizal (Myc+, R. irregularis) or non-mycorrhizal (Myc–) roots of Zea mays L. Root samples were harvested at 20 days post planting (dpp). Expression levels are shown relative to the constitutively expressed maize ubiquitin gene. Data are given as means ± standard deviation (SD) of three biological replicates. ** P < 0.01, * P < 0.05, Welch’s t test (Myc+ versus Myc–treatment).
MATERIALS AND METHODS
Preparation of two-compartment system for Iron-59 (59Fe) uptake assay
To examine the Fe uptake via mycorrhizal pathway, we developed a two-compartment culture system which spatially separates the root compartment (RC) and hyphal compartment (HC) (Fig. S1). HC is a cylindrical column (90 mm in height; 35 mm in diameter) containing the same soil as RC, and separated from RC by a three-layered barrier comprising an outer polyethylene filter (122 µm opening, PE-180, Sansho Co. ltd, Tokyo, Japan), a medial polyethylene mesh (1.5 mm in thickness; 2-mm opening to make an air gap to prevent capillarity action, Netlon protector C-16, Lite corporation, Tokyo, Japan), and an inner nylon filter (42 µm opening, N-No.330T, Tokyo Screen Co. ltd. Tokyo, Japan) (Fig. S1a). The inner filter prevents roots from penetrating into HC and allows mycorrhizal hyphae penetration. The bottom of HC is covered with a polystyrene dish (10 mm in height; 37 mm in diameter) to prevent the water in HC from flowing out (Fig. S1a). The gap between the outer polyethylene filter and the bottom dishes was sealed with a commercially available hot melt adhesive. The HC was introduced into a 500-mL polyethylene pot (120 mm in height; 100 mm max. diameter; two 6-mm holes in diameter at the bottom) from the initiation of cultivation. The bottom of HC was placed about 30 mm above the bottom of the pot. Maize plants were grown in RC, and 10 mL aqueous solution containing Iron-59 (III) chloride (59FeCl3) (925 kBq) was gently applied to each HC at 14 days post planting (dpp) using a pipette.
Plant cultivation
In 59Fe uptake analysis, soils and containers were prepared as follows. Akadama soil (tuff loam) (100 g, Setogahara Kaen, Gunma, Japan) was placed on the bottom of RC. About 1000 spores of Rhizophagus irregularis (formerly called Glomus intraradices DAOM 197198, Premier Tech, Riviere-du-Loup, Canada) was mixed thoroughly with upper-layer soil [Kanuma soil (weathered volcanic lapillus)/Ezo sand (small pumice)/Nippi soil (granular potting soil) (Nihon Hiryo, Tokyo, Japan) mixture (15:7:4, by weight)]. The total of 260 g upper-layer soil was placed on top of the Akadama soil. HC contained 52 g of the soil without fungal inoculant. Surface-sterilized seeds of maize (sweet corn, Kaneko Seeds, Co. ltd., Gunma, Japan) were sown in RC, and were watered every other day from the bottom by maintaining a water level less than 5 mm in depth. A single plant was left in each container and received 50 mL nutrient solution (1/2 strength Hoagland without Fe, pH 5.7) once a week from the bottom. HC were also watered (5–10 mL) every day from the top using a pipette when the soil surface was dried (Kanuma soil changes in color from dark yellow ocher to light yellow ocher when it is dried). Plants were cultivated in a growth chamber at 26°C with 16 h day/8 h night cycle.
Plants for gene expression analysis were cultivated using the same soil and the same pots as for 59Fe uptake analysis. Each pot contained 250 g of soil mix as an upper layer and about 1000 spores of R. irregularis. The plants were watered every other day from the bottom by maintaining a water level less than 5 mm in depth. No nutrient solution was added. The cultivation was conducted in a greenhouse with a temperature of 16 h day/8 h night cycle at 26°C day/23°C night.
Measurement of 59Fe radioactivity
Leaves (blades) of each position harvested at 44 dpp or soils from RC and HC were placed in test tubes. Soils of each compartment were homogenized by stirring and a portion (10–12 g) was subjected to measurement. 59Fe activities in each sample were quantified using a well-type NaI(Tl) scintillation counter (2480 WIZARD2, PerkinElmer, Massachusetts, USA). To detect the radioactivity of leaves, high-purity germanium detectors were used. Each leaf placed in test tubes was recovered, combined, dried for at least 3 d at 60°C, and subjected to gamma-ray spectrometry using germanium detectors (GAMMA-X(GMX) N-type coaxial HPGe, SEIKO EG&G, Tokyo, Japan).
Total RNA extraction and quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from mycorrhizal or non-mycorrhizal roots using NucleoSpin Plant RNA extraction kit according to the manufacturer’s instructions (Macherey-Nagel, Düren, Germany). The yield and purity of the RNA were determined spectrophotometrically (NanoDrop, Thermo Scientific, Wilmington, DE, USA). First-strand cDNA was synthesized from 100–290 ng of total RNA using PrimeScriptTM RT Master Mix (Perfect Real Time, Takara, Tokyo, Japan) according to the manufacturer’s instructions. Specific cDNA were amplified by SYBR Premix Ex Taq II (Takara) and gene-specific primers (Table S1) using real-time PCR (Thermal Cycler Dice, Takara). PCR reactions were one at 95°C for 30 s heat and 40 cycles of 95°C for 5 s and 60°C for 30 s. Specificity of each PCR product was assessed with the dissociation curve (95°C for 15 s, 60°C for 30 s, and 95°C for 15°C). The transcript levels of the target gene were calculated and expressed as fold expression in comparison to the constitutively expressed ubiquitin gene of maize (ZmUBQ, GRMZM2G118637) using the standard curve method.
RESULTS AND DISCUSSION
AM fungi delivered 59Fe to maize
We examined the Fe transport by AM fungi to maize using a two-compartment culture system (Fig. S1a and b). All leaves (blades and sheaths) of each plant were combined, dried and subjected to 59Fe determination. 59Fe was detected in the shoots of mycorrhizal plants, but not in non-mycorrhizal plants (Fig. ). 59Fe levels detected in the soils of RC were much lower than those of HC, and were similar between mycorrhizal and non-mycorrhizal treatments (Fig. S2), indicating that 59Fe did not move from HC to RC. These results suggested that AM fungi took up Fe from the soils and delivered it to the host, similar to the observation by Caris et al. (Citation1998), who also demonstrated that mycorrhizal peanut (Arachis hypogea L.) and sorghum (Sorghum bicolor L.) could take up59Fe via AM fungi.
Expression analysis of several genes involved in Fe homeostasis
To obtain insights into the possible mechanism of Fe uptake in mycorrhizal roots of maize, we examined the expression levels of 10 genes that are possibly involved in Fe uptake/responses. The selection criteria of 10 genes were as follows. (i) The transcript was observed in the genome-wide atlas of the transcription of Maize (eFP Browser, Winter et al. Citation2007; Sekhon et al. Citation2011). (ii) The gene was the ortholog or the close homolog of a functionally characterized gene of the other species. (iii) The gene was mainly expressed in roots. The details of the selection process are described in the Supporting Information (Gene information). The accession numbers of the genes, the expression levels in eFP Browser and the phylogenetic trees are provided in Table S2, and Fig. S3 and S4. Total RNA was isolated from roots at 20 dpp. Roots were also stained with wheat germ agglutinin-fluorescein isothiocyanate (WGA-FITC), confirming the successful colonization (Fig. S5). Quantitative RT-PCR (qRT-PCR) analyses showed that the symbiotic phosphate transporter ZmPT6, which expresses in mycorrhizal roots and is required for phosphate uptake via AM fungi (Willmann et al. Citation2013), was specifically expressed in the inoculated roots (Fig. ). The transcript levels of ZmNASa, ZmNASf and ZmNAATa were significantly elevated in mycorrhizal roots compared to non-mycorrhizal roots (Fig. ). However, the expression levels of ZmDMASa, ZmYS1, ZmIRTa, ZmIRTb and ZmIRO2a were similar between the two treatments. ZmIRO2b was not detected in our qRT-PCR (data not shown). Rice (Oryza sativa L.) IRO2 encodes a basic helix-loop-helix (bHLH) transcription factor which positively regulates various genes related to Strategy II (Ogo et al. Citation2007), and the expression of maize ortholog ZmIRO2 (Nozoye et al. Citation2013) is also strongly induced under Fe-deficient conditions. The soils used in this study (Kanuma soil, Akadama soil and Nippi soil) are rich in Fe; the leaves of neither non-mycorrhizal (Myc-) plants nor mycorrhizal (Myc+) plants showed any Fe-deficiency symptoms (Fig. S5a). Therefore, it is unlikely that ZmNASa, ZmNASf and ZmNAATa were induced by Fe deficiency. These results suggested that nicotianamine synthesis was transcriptionally up-regulated in mycorrhizal roots of maize, whereas DMA synthesis and its uptake in the form of Fe(III)-DMA might not be important for Fe uptake in mycorrhizal roots. Supporting this, a ys1 mutant inoculated with AM fungi showed an enhanced growth compared with non-inoculated ys1 (see supporting information for the materials and method, Fig. S6a), increasing the dry weight of shoots and roots 3.2 times and 2.6 times, respectively (Fig. S6b and c). Chlorophyll levels were increased in mycorrhizal plants (Fig. S7) and the total Fe contents in shoots and roots were also increased to 3.6 times and 1.6 times, respectively, as well as the increased accumulation of phosphorus (Fig. S8). Clark and Zeto (Citation1996a, Citation1996b) also reported similar results using acid and alkaline soils. We found that nicotianamine synthesis was likely to be up-regulated in maize mycorrhizal roots. Because it is thought that nicotianamine is not secreted but plays a role in the internal transport of Fe (von Wiren et al. Citation1999; Takahashi et al. Citation2003), nicotianamine synthesized in mycorrhizal roots may chelate Fe delivered by AM fungi within the roots or shoots.
Intriguingly, the transcript levels of oligopeptide transporter genes ZmOPT8a and ZmOPT8b in mycorrhizal roots were induced 194- and 62-fold, respectively (Fig. ). The OPT family is thought to have important roles in metal uptake and translocation (Bogs et al. Citation2003; Wintz et al. Citation2003; Cagnac et al. Citation2004; Stacey et al. Citation2007; Pike et al. Citation2009; Hu et al. Citation2012). In rice, OsOPT1, OsOPT3 and OsOPT4 transport Fe-nicotianamine conjugates when heterologously expressed in yeast fet3fet4 defective in Fe uptake (Vasconcelos et al. Citation2008). It is thus possible that ZmOPT8s are involved in Fe delivery in mycorrhizal roots. In mycorrhizal roots, highly branched fungal structures, arbuscules, in which exchange of resources occurs between the fungus and the host plant, are formed in the cortical cells. Arbuscules are surrounded by plants’ periarbuscular membranes, on which symbiotic phosphate and ammonium transporters are localized to take up the nutrients (Harrison et al. Citation2002; Kobae et al. Citation2010). If Fe uptake also occurs at arbuscules, it is possible that ZmOPT8s are localized on periarbuscular membrane and implicated in Fe uptake. Alternatively, ZmOPT8s may have a role in long-distance transport of Fe in mycorrhizal plants. Considering the broad substrate specificity of the OPT family (glutathione, peptides of 4–13 amino acids, phytochelatin, heavy metals; Lubkowitz Citation2011), ZmOPT8s may also have other roles in addition to Fe delivery in mycorrhizal roots.
SUPPLEMENTARY MATERIAL
The supplementary material for this article is available online from: http://dx.doi.org/10.1080/00380768.2014.949854
Supplemental Materials
Download PDF (1.3 MB)ACKNOWLEDGMENTS
This work was supported by the Ministry of Agriculture, Forestry and Fisheries of Japan [Genomics for Agricultural Innovation (PMI-0003)] and the Network of Centers of Carbon Dioxide Resource Studies in Plants (NC-CARP). We thank Shingo Hata, Haruhiko Inoue, Hiromi Nakanishi and Tomoko Nozoye, for useful discussion, Naoko Nishizawa for providing ys1 seeds, Hiroshi Moriyama (Nihon Hiryo Co. Ltd) for providing Nippi soil and Shigeo Kobayashi (Kaneko Seeds Co. Ltd) for providing maize seeds.
REFERENCES
- Bogs J, Bourbouloux A, Cagnac O, Wachter A, Rausch T, Delrot S 2003: Functional characterization and expression analysis of a glutathione transporter, BjGT1, from Brassica juncea: evidence for regulation by heavy metal exposure. Plant Cell Environ. 26, 1703–1711. doi:10.1046/j.1365-3040.2003.01088.x
- Cagnac O, Bourbouloux A, Chakrabarty D, Zhang MY, Delrot S 2004: AtOPT6 transports glutathione derivatives and is induced by primisulfuron. Plant Physiol. 135, 1378–1387. doi:10.1104/pp.104.039859
- Caris C, Hördt W, Hawkins HJ, Römheld V, George E 1998: Studies of iron transport by arbuscular mycorrhizal hyphae from soil to peanut and sorghum plants. Mycorrhiza. 8, 35–39. doi:10.1007/s005720050208
- Clark RB, Zeto SK 1996a: Iron acquisition by mycorrhizal maize grown on alkaline soil. J Plant Nutr. 19, 247–264. doi:10.1080/01904169609365120
- Clark RB, Zeto SK 1996b: Mineral acquisition by mycorrhizal maize grown on acid and alkaline soil. Soil Biol. Biochem. 28, 1495–1503. doi:10.1016/S0038-0717(96)00163-0
- Clark RB, Zeto SK 2000: Mineral acquisition by arbuscular mycorrhizal plants. J Plant Nutr. 23, 867–902. doi:10.1080/01904160009382068
- Conte SS, Walker EL 2011: Transporters contributing to iron trafficking in plants. Mol. Plant. 4, 464–476. doi:10.1093/mp/ssr015
- Curie C, Cassin G, Couch D, Divol F, Higuchi K, Le Jean M, Misson J, Schikora A, Czernic P, Mari S 2009: Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann. Bot. 103, 1–11. doi:10.1093/aob/mcn207
- Harrison MJ, Dewbre GR, Liu J 2002: A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell. 14, 2413–2429. doi:10.1105/tpc.004861
- Hu YT, Ming F, Chen WW, Yan JY, Xu ZY, Li GX, Xu CY, Yang JL, Zheng SJ 2012: TcOPT3, a member of oligopeptide transporters from the hyperaccumulator Thlaspi caerulescens, is a novel Fe/Zn/Cd/Cu transporter. PLoS ONE. 7, e38535. doi:10.1371/journal.pone.0038535
- Kobae Y, Tamura Y, Takai S, Banba M, Hata S 2010: Localized expression of arbuscular mycorrhiza-inducible ammonium transporters in soybean. Plant Cell Physiol. 51, 1411–1415. doi:10.1093/pcp/pcq099
- Kobayashi T, Nishizawa NK 2012: Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol. 63, 131–152. doi:10.1146/annurev-arplant-042811-105522
- Lubkowitz M 2011: The oligopeptide transporters: a small gene family with a diverse group of substrates and functions? Mol Plant. 4, 407–415. doi:10.1093/mp/ssr004
- Marschner H 2012: Mineral Nutrition of Higher Plants. 3rd ed. Academic, London.
- Nozoye T, Nakanishi H, Nishizawa NK 2013: Characterizing the crucial components of iron homeostasis in the maize mutants ys1 and ys3. PLoS One. 8, e62567. doi:10.1371/journal.pone.0062567
- Ogo Y, Itai RN, Nakanishi H, Kobayashi T, Takahashi M, Mori S, Nishizawa NK 2007: The rice bHLH protein OsIRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions. Plant J. 51, 366–377. doi:10.1111/j.1365-313X.2007.03149.x
- Pike S, Patel A, Stacey G, Gassmann W 2009: Arabidopsis OPT6 is an oligopeptide transporter with exceptionally broad substrate specificity. Plant Cell Physiol. 50, 1923–1932. doi:10.1093/pcp/pcp136
- Sekhon RS, Lin H, Childs KL, Hansey CN, Buell CR, de Leon N, Kaeppler SM 2011: Genome-wide atlas of transcription during maize development. Plant J. 66, 553–563. doi:10.1111/j.1365-313X.2011.04527.x
- Smith SE, Read DJ 2008: Mycorrhizal Symbiosis. 3rd ed. Academic Press, Inc., San Diego, CA.
- Stacey MG, Patel A, McClain WE, Mathieu M, Remley M, Rogers EE, Gassmann W, Blevins DG, Stacey G 2008: The Arabidopsis AtOPT3 protein functions in metal homeostasis and movement of iron to developing seeds. Plant Physiol. 146, 589–601. doi:10.1104/pp.107.108183
- Takahashi M, Terada Y, Nakai I, Nakanishi H, Yoshimura E, Mori S, Nishizawa NK 2003: Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. Plant Cell. 15, 1263–1280. doi:10.1105/tpc.010256
- Vasconcelos MW, Li GW, Lubkowitz MA, Grusak MA 2008: Characterization of the PT clade of oligopeptide transporters in rice. Plant Gen. 1, 77–88. doi:10.3835/plantgenome2007.10.0540
- von Wirén N, Klair S, Bansal S, Briat JF, Khodr H, Shioiri T, Leigh RA, Hider RC 1999: Nicotianamine chelates both FeIII and FeII. Implications for metal transport in plants. Plant Physiol. 119, 1107–1114. doi:10.1104/pp.119.3.1107
- Willmann M, Gerlach N, Buer B, Polatajko A, Nagy R, Koebke E, Jansa J, Flisch R, Bucher M 2013: Mycorrhizal phosphate uptake pathway in maize: vital for growth and cob development on nutrient poor agricultural and greenhouse soils. Front Plant Sci. 4, 533. doi:10.3389/fpls.2013.00533
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ 2007: An “electronic fluorescent pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE. 2, e718. doi:10.1371/journal.pone.0000718
- Wintz H, Fox T, Wu YY, Feng V, Chen W, Chang HS, Zhu T, Vulpe C 2003: Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J. Biol. Chem. 278, 47644–47653. doi:10.1074/jbc.M309338200
