Figures & data
1 1,3,5-Cyclohexatriene and [5]radialene sub-units, as found in C60 (double bonds are emitted for clarity)
![1 1,3,5-Cyclohexatriene and [5]radialene sub-units, as found in C60 (double bonds are emitted for clarity)](/cms/asset/e552cee0-38b9-4ac6-9fac-5a1f90cc9d75/ymst_a_1198114_f0001_c.jpg)
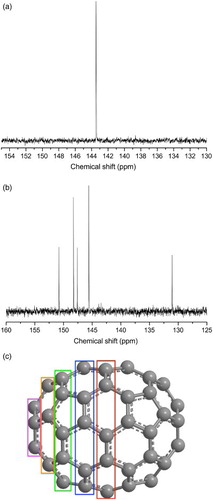
2 13C nuclear magnetic resonance (NMR) spectra of C60 a and C70 b. Every carbon environment in C60 is identical (all are equally strained in the spherical structure), while there are five environments in C70, corresponding to each successive, increasingly strained ‘layer’ of carbon atoms away from the equatorial plane c. NMR spectra were taken in carbon disulphide (CS2) on a Bruker AVIII 400MHz machine
3 The absorption spectra of the fullerenes change as the size of the conjugated system increases: with slight variations depending on solvent, solutions of C60 are an intense purple colour a, C70 red like wine b and C84 a green-yellow. (All above solutions in toluene). Generally speaking, the gap between the highest occupied molecular orbital and the lowest unoccupied molecular orbital decreases with increasing cage size leading to optical absorptions of lower energy, i.e. longer wavelengthCitation7

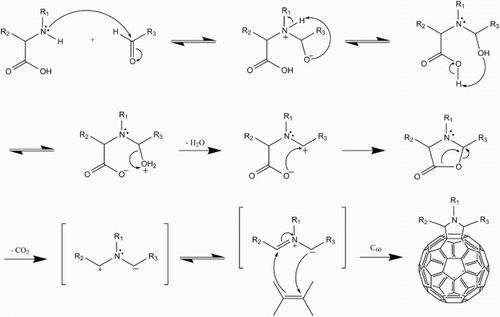
4 Amination mechanism of C60. The first step is single-electron transfer (a fast process) to produce the C60 anion radical which has a characteristic green colour.Citation23 Radical recombination gives a zwitterion which can be stabilised by proton transfer to give the final product (a slow process giving a brown solution)
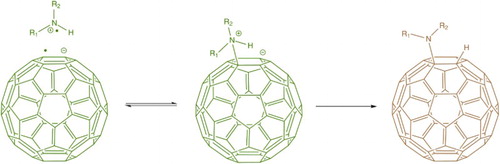
5 Prato reaction mechanism (after ScarelCitation29). Initial attack of the aldehyde/ketone's polar carbonyl group by the nitrogen lone pair of the amino acid leads to the expulsion of water, before decarboxylation gives the reactive ylide intermediate
6 Possible second addend addition sites (adapted after Citation31). There are three sites in the same hemisphere as the initial site of addition, giving rise to cis-regioisomers ,
and
; two equatorial sites, e' and e”; and four addition sites in the other hemisphere, giving trans-regioisomers
,
,
and
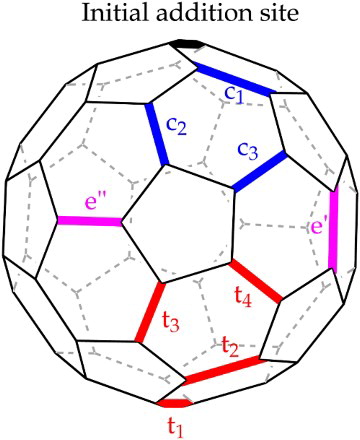
7 Bingel reaction mechanism. Deprotonation by the strong base gives the nucleophilic malonate anion which then attacks the [6,6] bond. Bromine is then expelled as cyclisation is completed
![7 Bingel reaction mechanism. Deprotonation by the strong base gives the nucleophilic malonate anion which then attacks the [6,6] bond. Bromine is then expelled as cyclisation is completed](/cms/asset/c69eb956-ba6d-444a-84a8-e7ea6342a972/ymst_a_1198114_f0007_b.gif)
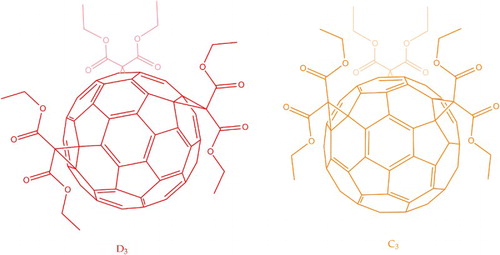
8 C60 diethyl malonate tris-adducts. Addition at the trans-3 positions imparts symmetry and produces a red solution; at the equatorial positions,
symmetry and an intense orange solution
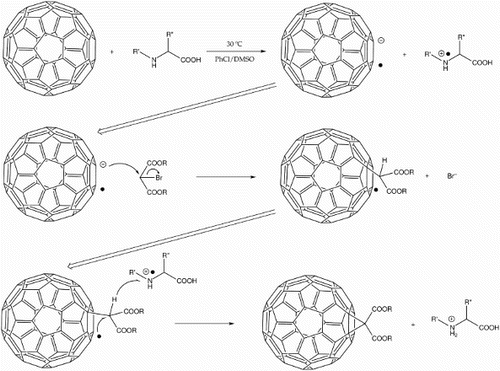
10 C60 Bingel reaction in the presence of dimethyl sulphoxide (DMSO), with basic catalyst replaced by amino acid (adapted from Jin et al.Citation42). DMSO facilitates single-electron transfer from the amino acid to the fullerene to give the radical cation and radical anion respectively. The fullerene anion then attacks a brominated active methylene, expelling the bromine and forming a fullerenyl radical. Interaction between this and the amino acid radical cation from the first step then finalises cyclisation to give the desired methanofullerene

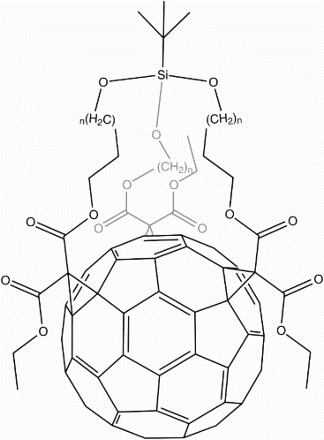
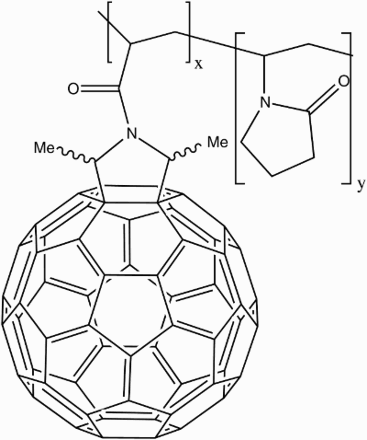
![12 Dendro[60]fullerene mono-adduct](/cms/asset/832ead3d-979a-41fb-9468-99776ea8dc9c/ymst_a_1198114_f0012_b.gif)
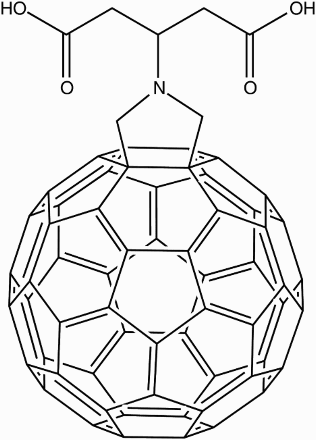
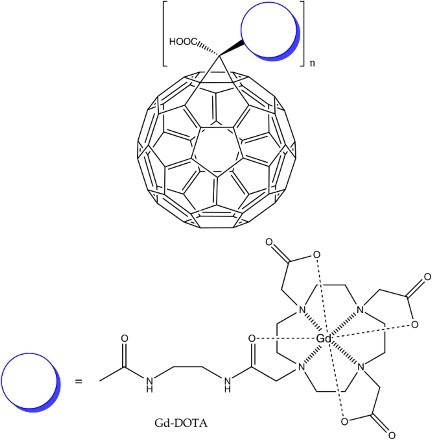
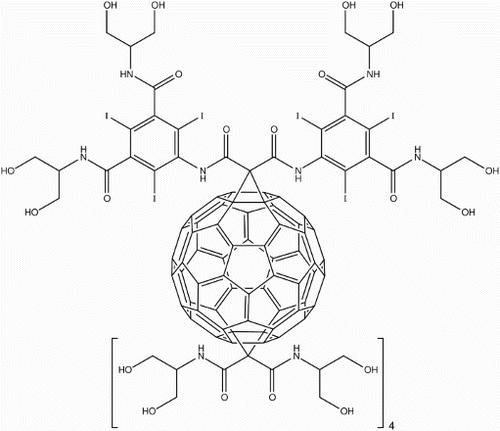
![16 First generation water-soluble dendro[60]fullerene](/cms/asset/883dd02d-c52c-4220-aa29-529fbb19a380/ymst_a_1198114_f0016_b.gif)