Figures & data
Table 1. Commercially approved protein pharmaceuticals produced via spray-drying.
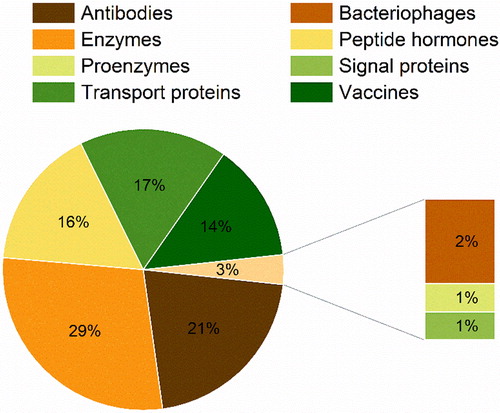
Figure 3. Overview of the published studies by year: (a) total number and (b) used class of protein pharmaceuticals.
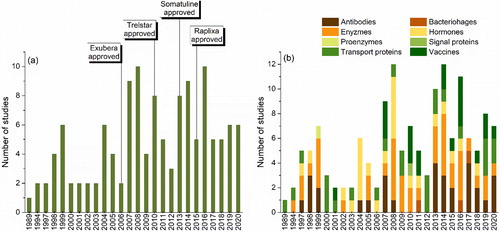
Figure 4. Classes of excipients used in the formulation of protein pharmaceuticals via spray-drying.
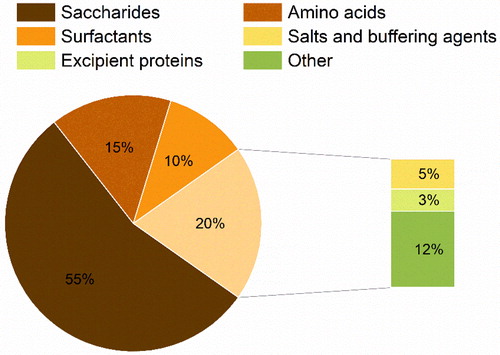
Figure 5. Sub-categorization of the saccharides used in the formulation of protein pharmaceuticals for spray-drying.
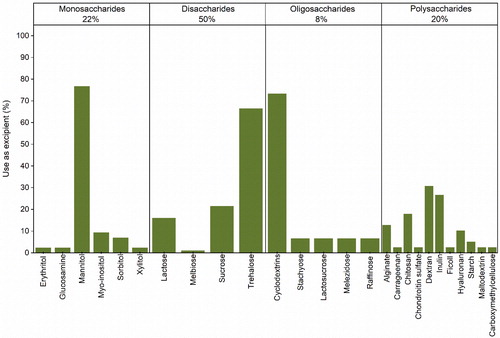
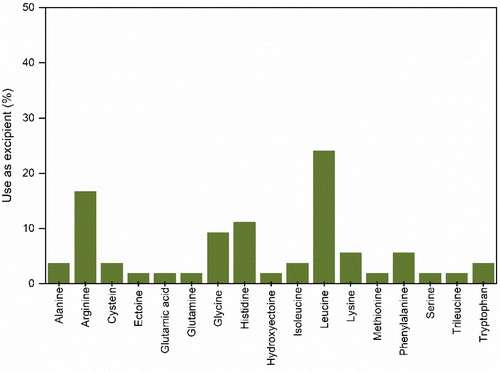
Figure 9. Proteins used as excipients in the formulation of protein pharmaceuticals for spray-drying.
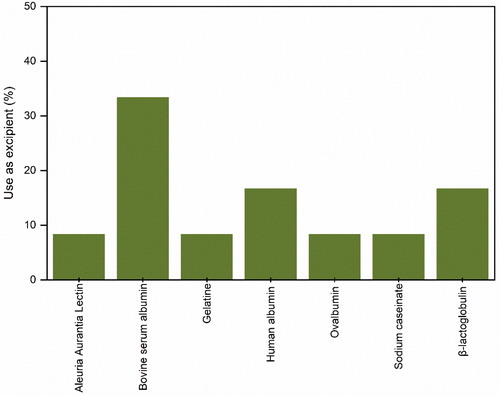
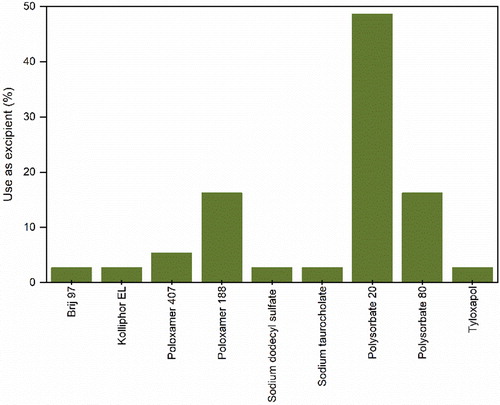
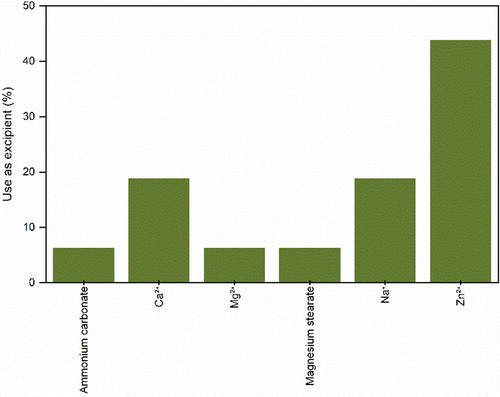
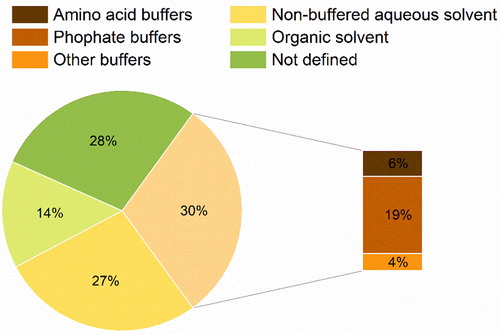
Figure 10. Sub-categorization of other various excipients used in the formulation of protein pharmaceuticals for spray-drying.
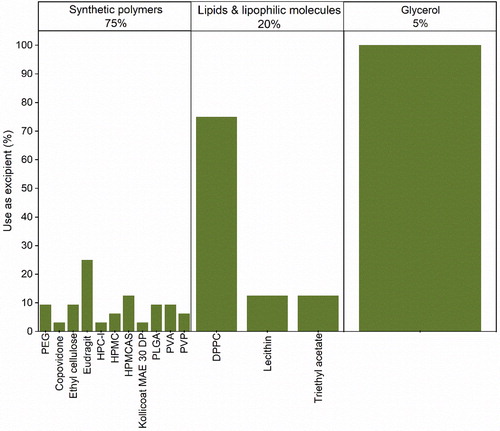
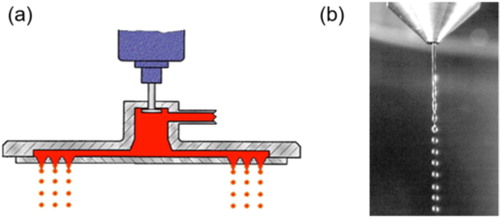
Figure 13. Overview of the DROPPO® system (a) schematic design of the die-head and (b) droplet formation on an individual jet.