Figures & data
Figure 1. miRNAs regulate the intestinal epithelium homeostasis by altering IEC survival, proliferation, migration, and cell-to-cell interaction. In this model, the levels of mucosal miRNAs are increased in the intestine after exposure to various pathological stresses. Increased miRNAs inhibit expression of their target genes by decreasing stability and translation of the mRNAs, thus leading to disruption of the gut epithelium homeostasis. RNA binding proteins (RBPs) such as HuR and CUGBP1 can interact with miRNAs including miR-195 and miR-222 and modulate their regulatory functions antagonistically or synergistically.
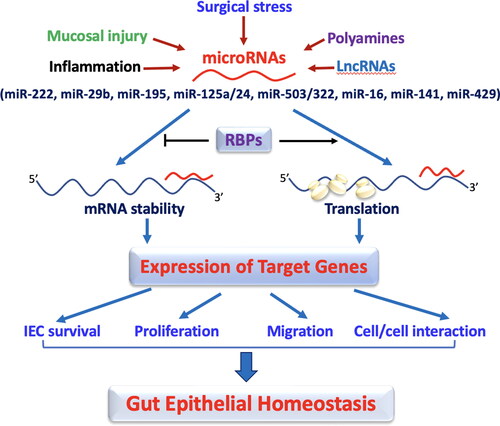
Table 1. Functions and targets of miRNAs in the intestinal epithelium.
Figure 2. LncRNA H19 disrupts the intestinal defense and barrier function via distinct mechamisms. Induced levels of mucosal H19 in the intestine by septic stress and mucosal inflammation repress expression of tight junctions (TJs) and adherens junctions (AJs), impair autophagy, and disrupt function of Paneth cells (PCs) and goblet cells. H19 inhibits expression of ZO-1 and E-cadherin posttranscriptionally through release of miR-675 embedded in H19 exon 1, and this inhibition is prevented by HuR.
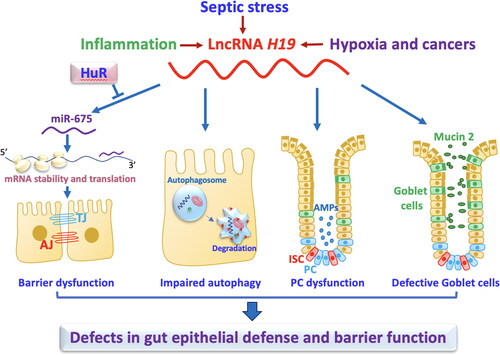
Table 2. Function of lncRNA in the intestinal epithelium.
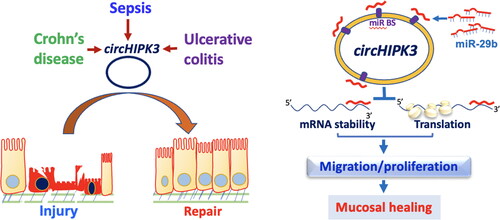
Figure 3. Model proposed to explain the influence of circHIPK3 upon intestinal epithelial repair after wounding. The levels of circHIPK3 are markedly altered in the intestinal mucosal tissues from patients with sepsis, Crohn’s disease, and ulcerative colitis. Increased circHIPK3 plays an essential role in intestinal mucosal repair after injury via interaction with miR-29b.