Figures & data
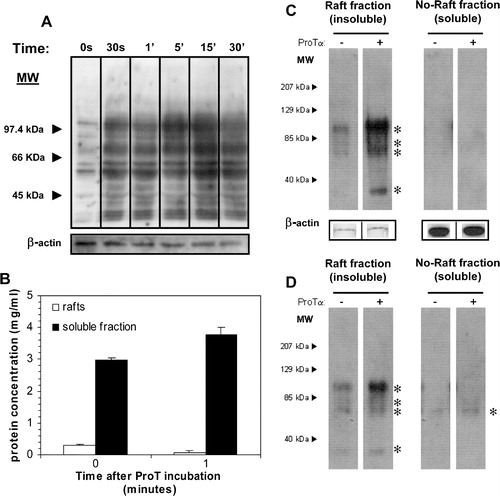
Figure 1. ProTα receptor is located in rafts microdomains. After BS3-mediated crosslinking of biotin-ProTα to surface receptors on human lymphoblasts (50×106) cell lysis was carried out in TKM buffer containing 0.5% TX-100. The cell lysate was then adjusted to 40% sucrose and subjected to equilibrium density gradient centrifugation in a SW55Ti rotor. After a overnight centrifugation at 200,000×g, 11 fractions (0.45 ml/each) were collected from top to bottom. Serial dilutions of the fractions were dotted on and analysed for total protein (BCA assay; a), alkaline phosphatase activity (BCIP/NBT-based assay; a and b), and the presence of ProTα receptor (b), CD59 (c) and CD71 (c). Films were scanned, subjected to densitometry, and data shown as raw arbitrary units (b) or expressed as a percentage of the total amount for the respective antigen (a, c). (d) Analysis by SDS-PAGE of pooled fractions obtained after running a raft membrane extract from human lymphoblasts through a ProTα affinity column.
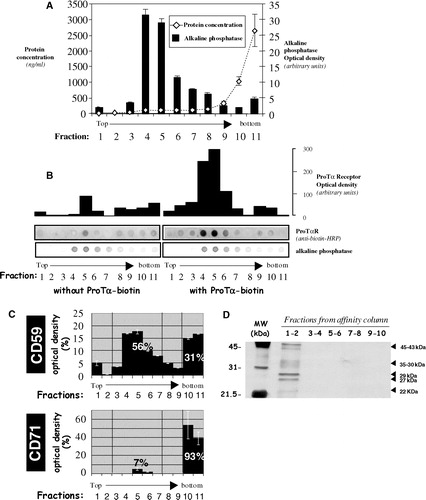
Figure 2. Improvement of a CTB-HRP-based method for the specific depletion of raft proteins. Lymphoblasts were separated in different samples, incubated with various amounts of cholera toxin (CTB; 0.01, 0.05, 0.1 or 1 µg/ml) for 30 min at 37°C and then washed several times with HBSS+. Next, cells were placed in 1 ml HBSS+ and the 3,3′-diaminobenzidine (DAB) crosslinker, either at 0.5 or 0.1 mg/ml, added in the presence of different concentrations of H2O2 (0.01, 0.05 or 0.1%) for 45 min at 4°C. After this incubation step, lymphoblasts were washed twice with cold HBSS+ and cell lysis performed with TKM/0.5% TX-100 for 30 min on ice. A postnuclear supernatant was obtained after centrifugation at 13,000 rpm for 15 min (4°C) to eliminate, amongst others, nuclei and rafts proteins. Soluble proteins (non raft proteins) were dotted on a nitrocellulose membrane (HybondECL, Amersham-Biosciences Europe, GmbH) and the presence of CD59 (raft marker) and CD71 (non raft marker) analysed as in by Immunoblotting combined with ECLPlus (Amersham-Biosciences Europe, GmbH). Initial expression of both CD59 and CD71 was evaluated in non-treated cells.
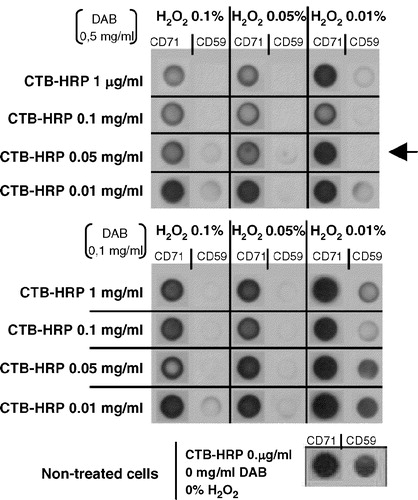
Figure 3. Raft protein crosslinking and depletion from lymphoblasts leads also to a ProTα receptor removal. Aliquots of 2×106 PHA-activated cells were cross-linked with 20 µl mM 1 BS3, in the absence or presence of 15 µM biotin-ProTα. To eliminate raft proteins all samples were treated with CTB-HRP (0.05 µg/ml) and incubated (+) or not (−) with DAB (0.5 µg/ml) in the absence (−) or presence (+) of H2O2 (0.01%) as indicated. After lysis and centrifugation, samples were dotted on a nitrocellulose membrane and the presence of ProTα receptor (a) and CD59 (b) detected with anti biotin-HRP and anti CD59 + GAM-HRP, respectively.
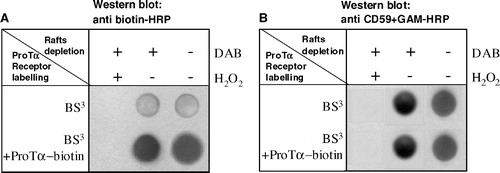
Figure 4. Flow cytometry analysis of the ProTα receptor association with raft regions on human lymphoblasts. Cells (2×106) were cross-linked with BS3 in the absence (to check for background fluorescence; see also , right) or the presence of 15 µM of biotin-ProTα, and then treated with 1% TX-100, 10 mM MβCD or both as indicated in Material and Methods. After paraformaldehyde fixation, ProTα receptor and CD71 expression was revealed by staining with streptavidin-PE and anti CD71-FITC, respectively. In order to know the percentage of CD71+ cells and to place the histogram marker as shown, a FITC-labelled IgG2aκ isotype antibody was used as a negative control. Data acquisition was done on a Becton Dickinson FACScalibur flow cytometer, while WinMDI software was used to analyse the data. This experiment is representative of several with similar results.
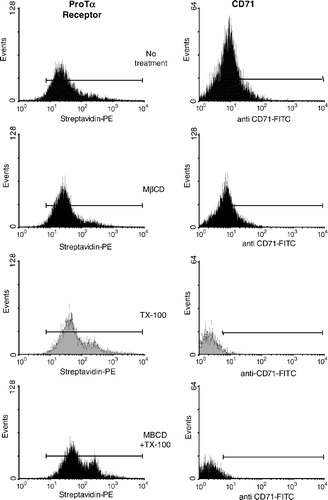
Figure 5. High resistance to non ionic detergent extraction is consistent with an in vivo location of ProTα receptor within rafts. Cells from samples described in were analysed by flow cytometry for streptavidin-PE (ProTa receptor) immunofluorescence (a) and for forward and right-angle light scattering (b). In (a), negative control cells (right) were not incubated with biotin-ProTα in order to show the non specific binding of streptavidin-PE. Dot plot (forward versus right-angle scattering) and histograms (CTB-Alexa 488 versus events) shown in figure (c) represent human PHA-lymphoblasts from a different donor treated with or without 1% TX-100 and stained with CTB-Alexa 488 to reveal GM1 ganglioside expression.
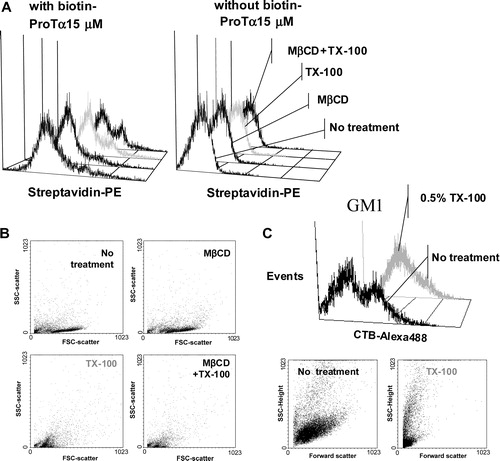
Figure 6. ProTα receptor and CD59 show a similar distribution pattern on plasma membrane from human lymphoblasts. Aliquots of 2×106 cells were cross-linked with 1 mM BS3 in the presence (figure) or absence (not shown) of 15 µM biotin-ProTα. Lymphoblasts were incubated with streptavidin-PE to reveal ProTα receptor expression (red) and also, in order to induce either raft or non-raft proteins clustering, stained with anti CD59 or anti CD71 mAbs respectively at 4°C and incubated at RT (to allow free lateral movements of proteins) with a FITC-labelled anti-IgG Ab to cross-link any antibody on the cell surface. Both CD59 and CD71 expression are shown in green, while colocalization level (merged) of any of these markers with ProTα receptor is presented in yellow.
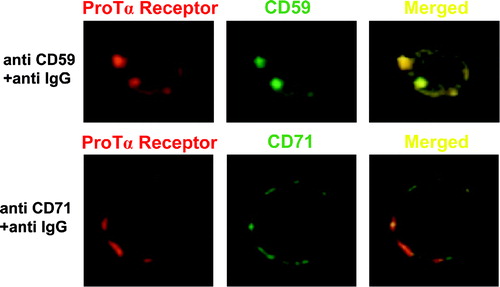
Figure 7. ProTα induces a tyrosine phosphorylation event confined to rafts in lymphoblasts. (a) Cells were incubated or not with 5 µg/ml of ProTα for different times and their lysates run on a 7.5% SDS-PAGE electrophoresis gel, transferred to a PVDF membrane and the presence of phosphotyrosines revealed with an anti-phosphotyrosine mAb (clone PY20, BD-Biosciences) in combination with GAM-HRP and ECLPlus (Amersham Biosciences Europe, GmbH). (b) Proteins from cells incubated or not with 5 µg/ml of ProTα for 1 min at 37°C were extracted as indicated in . Raft fractions (3–6) and soluble fractions (10–11) were pooled and protein concentration determined (BCA, Pierce Biotechnology, Inc). Identical amounts of protein (2 µg; c) and volume (10 µl; d) from both pooled insoluble and soluble fractions were run on a 7.5% SDS-PAGE gel and detection of phosphotyrosines performed as before. In a and c protein loading controls were established after membrane stripping and incubation with an anti β-actin mAb.