Figures & data
Table 1. Dose-response for the in vitro effects of arsenic in primary cells.
Table 2. Benchmark dose ranges for genes with a statistically significant dose-response trend in primary urothelial cells from most subjects after treatment with arsenite, MMAIII, and DMAIII (trivalent) mixtures.
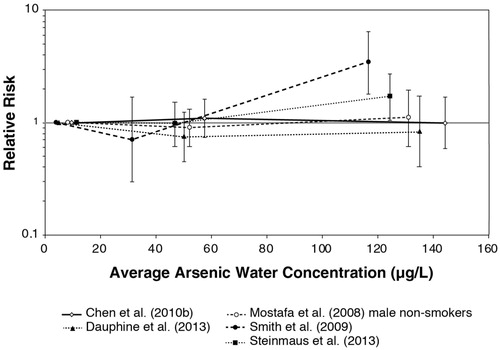
Table 3. Studies of low-level arsenic exposureTable Footnote* included in the main evaluation of dose-response in the current study in comparison to Lynch et al. (Citation2017a) and non-ecological studies in Lamm et al. (Citation2015) and Tsuji, Alexander et al. (Citation2014).
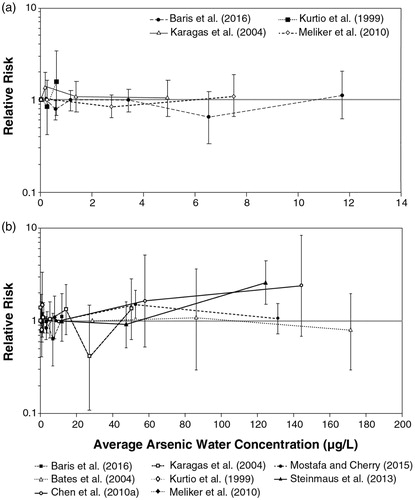
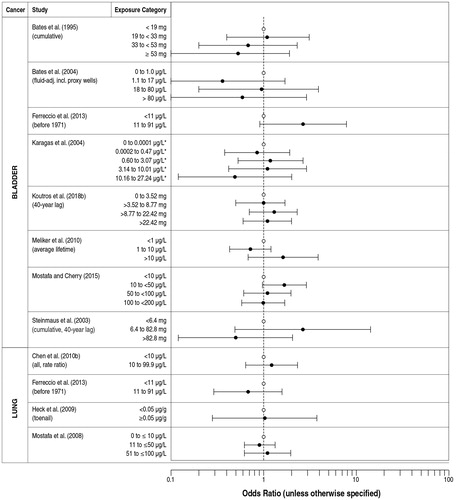
Table 4. Studies included in the current dose-response evaluation of arsenic water concentration and bladder cancer risk.
Table 5. Studies included in the current dose-response evaluation of arsenic water concentration and lung cancer risk.
Table 6. Results of epidemiological studies of low-level arsenic exposure and risk of bladder or lung cancer among never smokers. Results in italics are not directly relevant to the dose-response assessment of low-level drinking water arsenic and bladder or lung cancer risk but are included for completeness.
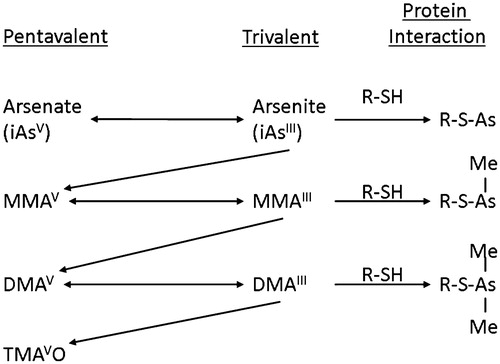
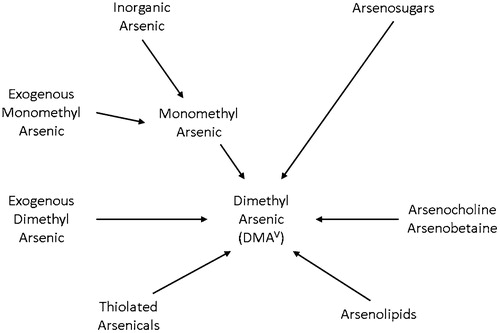
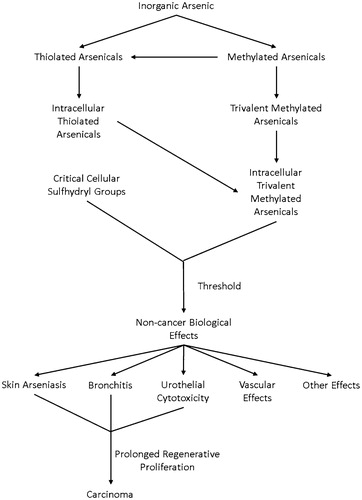
Table 7. Convergence of evidence: inorganic arsenic.