Figures & data
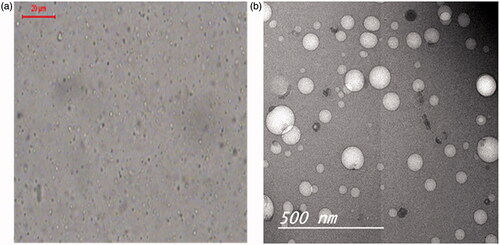
Table 1. Composition and characterization of the prepared bosentan RCRPC based on central composite design.
Figure 2. Aerodynamic parameters of the optimized bosentan RCRPC, drug solution and drug suspension after nebulization in a TSI.
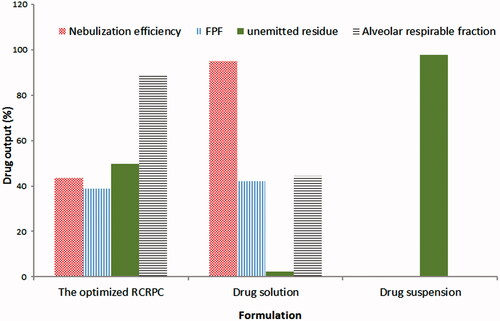
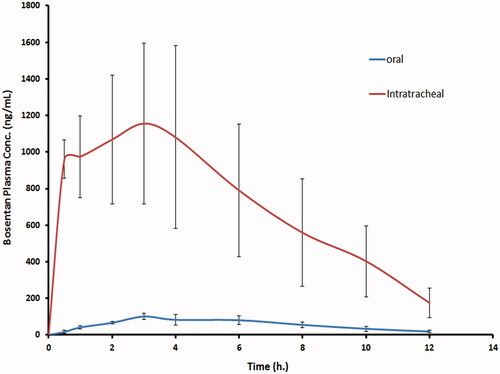
Figure 3. The mean bosentan concentrations in plasma of rats after intratracheal delivery of the optimized bosentan RCRPC compared to the oral administration of bosentan suspension.