Figures & data
Figure 1 Chemical structures of compounds tested in the hGAD65 in vitro. assay. Glc; β-d-glucose; Rha, α-l-rhamnose; Arap, α-l-arabinose(pyranose); Araf, α-l-arabinose (furanose).
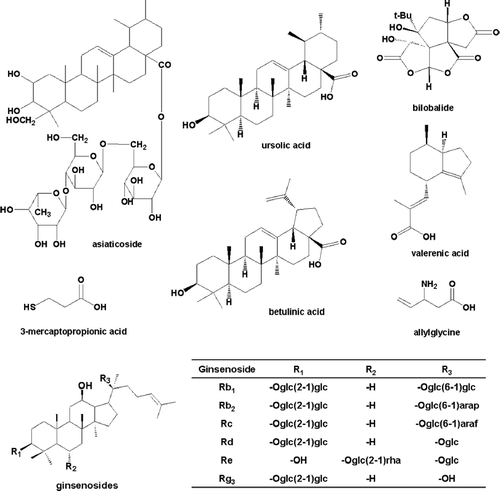
Figure 2 The pMAL-c2x/hGAD65 fusion construct. The hGAD65 coding sequence was fused in-frame with the DNA sequence for maltose binding protein, malE, under the control of “Tac” promoter.
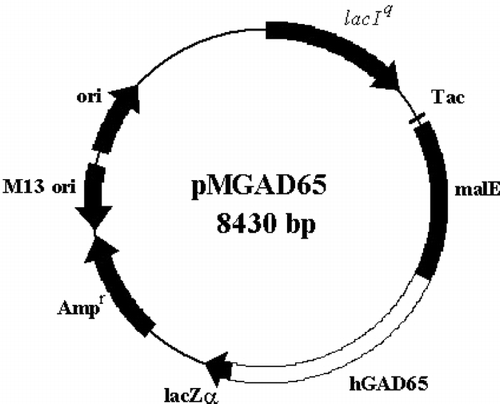
Figure 3 Growth curves of E. coli. containing either MBP-hGAD65 or GST-hGAD65 fusion proteins, expressed as the absorbance at 600 nm.
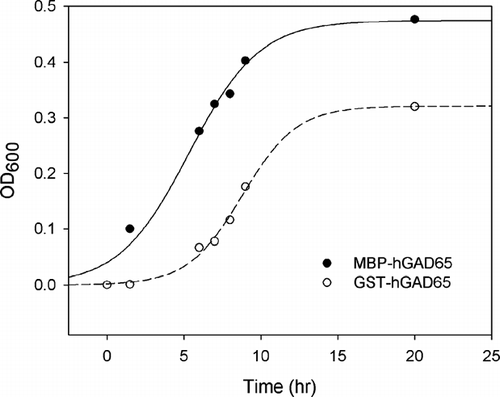
Figure 4 SDS-PAGE gels stained with silver reagent to detect GST-hGAD65 (fractions 2–5) and MBP-hGAD65 (fractions 1–4) fusion proteins collected after purification on glutathione sepharose and amylose columns, respectively. Molecular mass markers are outlined in the center. The GST-hGAD65 fusion appears as a dark band below the 97.4 kDa, and the MBP-hGAD65 fusion migrates just above it. The GST and MBP fractions were run on two different gels. They were handled similarly and stained on the same day. Note the presence of a large number of contaminating bands in the GST fractions.
Figure 5 Linear regression analysis (± 95% confidence intervals) of the inhibitory effect of 3-MPA on in vitro. hGAD65 activity, represented as a percent of the control. IC50 = 12.3 μ M (n = 3 for each concentration tested).
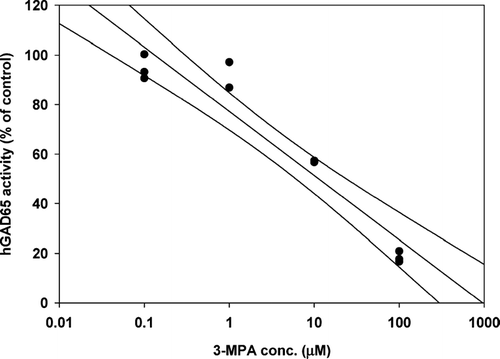
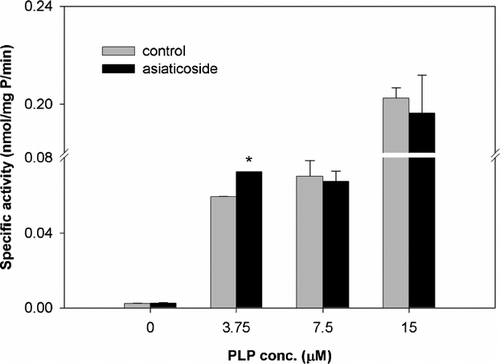
Table 1 Mean percent change (± SD) of in vitro. hGAD65 activity by phytochemicals and a ginseng extract compared with 3-MPA.