Figures & data
Figure 1 Scheme of metabolic conversion of daunorubicin to its C13-hydroxymetabolite daunorubicinol. The cytosolic reductases involved in this process are CR (EC 1.1.1.184), AKR1C2 (EC 1.3.1.20), and AKR1A1 (EC 1.1.1.2).
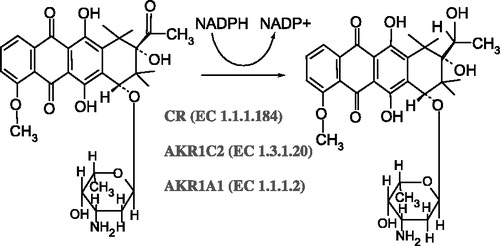
Figure 2 The activities of the three cytosolic reductases measured at their optimal pH. The first column represents the sum of activities of CR and AKR1C2 with the pH optimum at 6.0. The activity of AKR1A1 could be measured separately at pH 8.5.
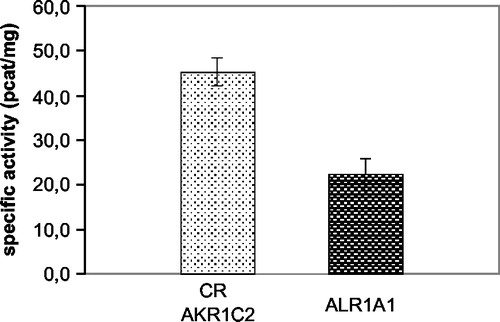
Figure 3 The inhibition curves of quercitrin (A) and flufenamic acid (B) at pH 6.0 and pH 7.4 for comparison. The participation of CR and AKR1C2 to the total reductase activity was calculated to be 3:1 at pH 6.0 (optimal) and 5:1 at pH 7.4 (physiological). The IC50 values at pH 6.0 were estimated to be 5.45 ± 1.37 μM for quercitrin and 3.68 ± 1.58 μM for flufenamic acid.
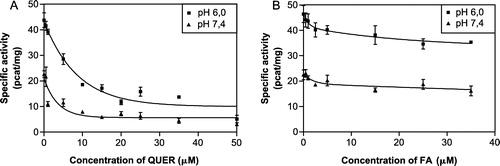
Figure 4 Michaelis-Menten kinetics of daunorubicin reduction at pH 6.0. The kinetic parameters calculated from the curve were:
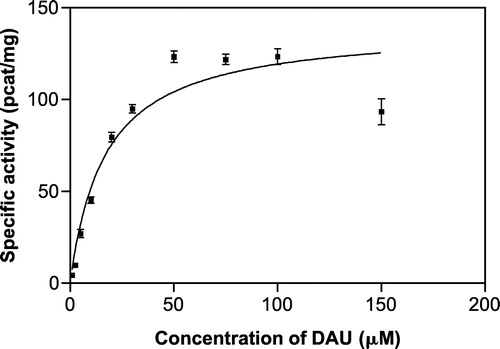
Figure 5 Michaelis-Menten kinetics of daunorubicin reduction at pH 6.0 inhibited by quercitrin (A) and flufenamic acid (B) at two different concentrations by comparison with the non-inhibited reduction.
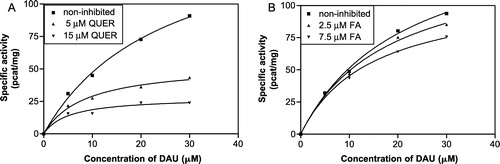
Table I. IC50 values, Ki constants and inhibition types of quercitrin and flufenamic acid for rabbit liver carbonyl reductase and aldo-keto reductase AKR1C2, respectively.
Figure 6 Linearization of the inhibition data according to Lineweaver and Burk for of daunorubicin reduction by quercitrin and flufenamic acid (at pH 6.0).
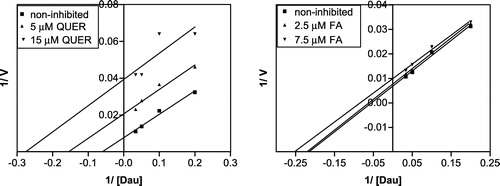
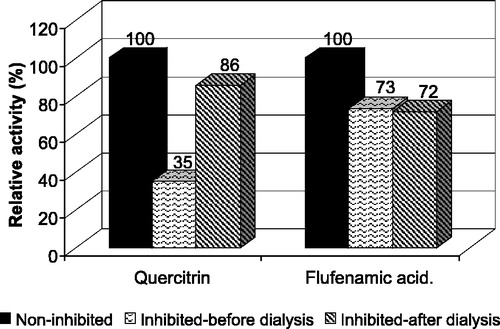
Figure 7 Inhibition reversibility assay. The reduction was inhibited by 10 μM quercitrin and 5 μM flufenamic acid to 35% and 73% of control, respectively. After 12 h of dialysis in PBS the inhibition by quercitrin was reversed and the activity reached 86% of control. In the case of flufenamic acid no significant changes in activity were observed due to dialysis suggesting reversible inhibition of CR by quercitrin and irreversible inhibition of AKR1C2 by flufenamic acid.