Figures & data
Table I. The angiostatic response between 5FU, THS, physical mixtures of THS and 5FU versus the THS-5FU codrug at varying dosages. Angiostatic effect is determined by the presence of an avascular zone. An avascular zone of ≥4 mm at the site of implantation indicates a positive response, otherwise a negative response is indicated.
Figure 1 Synthesis of 3α-O, 21-O-(dichloroformyl)-5β-pregnane-17-ol-20-one (5) from Reichstein's Substance S (1).
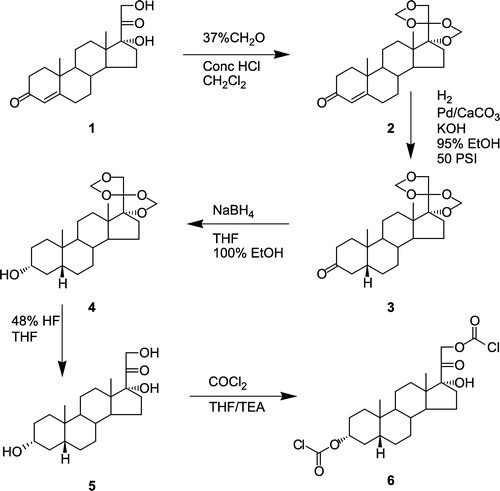
Figure 2 Coupling of 3α-O, 21-O-(dichloroformyl)-5β-pregnane-17-ol-20-one (6) and N1,N3-Bis-dihydroxymethyl 5-fluorouracil (8) to produce O3-(N1-methyloxycarbonyl-5-fluorouracil)5-β-pregnane-17-ol-20-one (9),O21-(N1-methyloxycarbonyl-5-fluorouracil)5-β-pregnane-17-ol-20-one (10), and O3-, O21-Di(N1-methyloxycarbonyl-5-fluorouracil)5-β-pregnane-17-ol-20-one; (THS-BIS-5FU) (11).
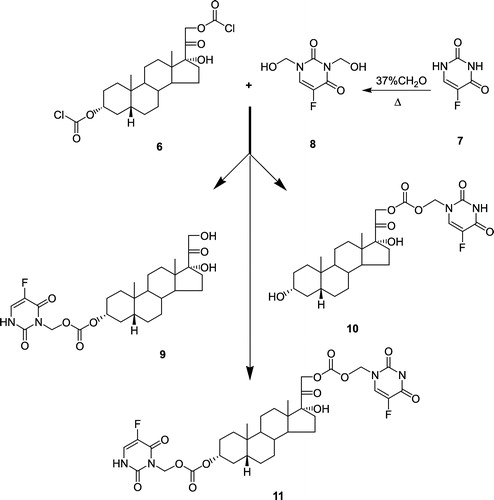
Figure 3 Proposed hydrolysis of the model THS-BIS-5FU codrug (11). One molar equivalent of codrug should generate 1 molar equivalent of THS, 2 molar equivalents of 5FU, and 2 molar equivalents of both formaldehyde and carbon dioxide.
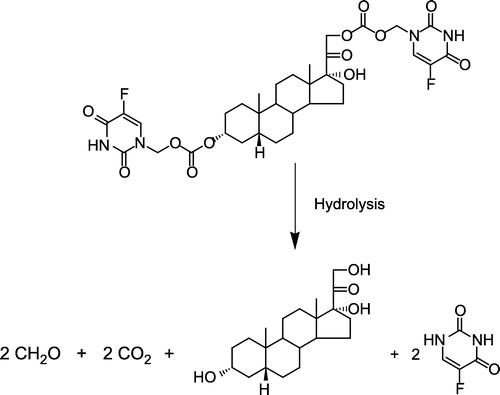
Figure 4 The appearance of 5FU (•) and THS (▴) in molar concentration from the degradation of THS-BIS-5FU codrug (▪) in phosphate buffer (0.1 M, pH 7.4).
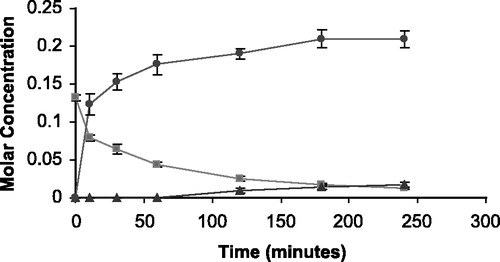
Figure 5 The appearance of 5FU (•) and THS (▴) in molar concentration from the degradation of THS-BIS-5FU codrug (▪) in human serum.
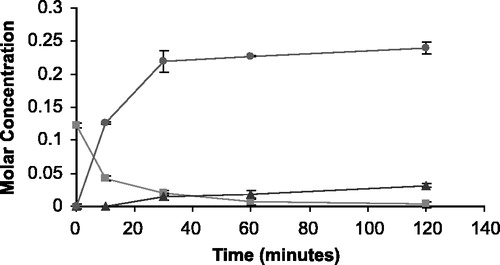
Figure 6 The appearance of 5FU (•) and THS (▴) in molar concentration from the degradation of THS-BIS-5FU codrug (▪) in bovine vitreous humor.
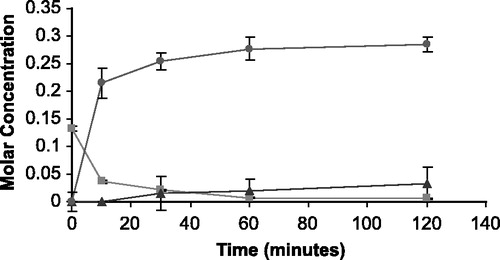
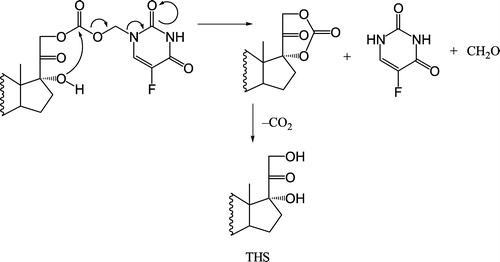
Figure 8 Chemical structure of a proposed THS carbonate ester, the cyclic intermediate formed from the hydrolysis of the O3α-, O21-di-(N1-methyloxycarbonyl-2, 4-dioxo-5-fluoropyrimidinyl)17α- hydroxy-5β-pregnan-20-one codrug [THS-BIS-5FU] (11), and the degradation of the cyclic intermediate to form THS.
![Figure 8 Chemical structure of a proposed THS carbonate ester, the cyclic intermediate formed from the hydrolysis of the O3α-, O21-di-(N1-methyloxycarbonyl-2, 4-dioxo-5-fluoropyrimidinyl)17α- hydroxy-5β-pregnan-20-one codrug [THS-BIS-5FU] (11), and the degradation of the cyclic intermediate to form THS.](/cms/asset/78f7df48-a670-4e4c-92ab-65f9e745224f/ienz_a_122014_f0008_b.jpg)
![Figure 9 Proposed hydrolysis products, based upon 1H-NMR spectrum of the isolated hydrolysis intermediate O3α-, O21-di-(N1-methyloxycarbonyl-2, 4-dioxo-5-fluoropyrimidinyl)17α- hydroxy-5β-pregnan-20-one codrug [THS-BIS-5FU] (11).](/cms/asset/32c10d1d-6d2f-45d0-bc93-522e705af7c7/ienz_a_122014_f0009_b.jpg)