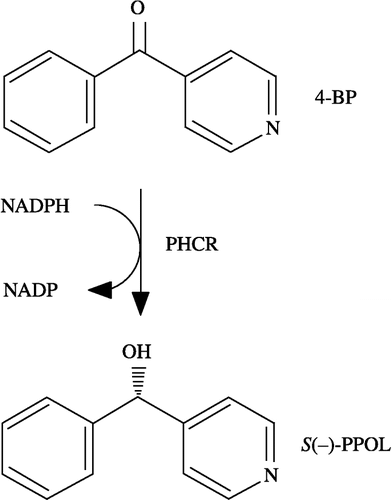
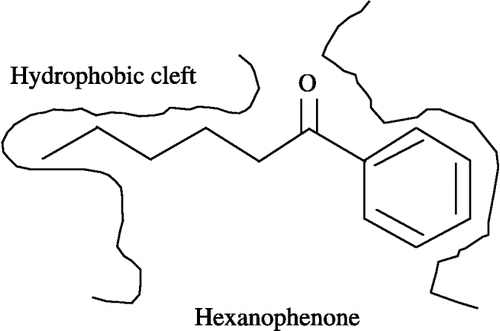
Figures & data
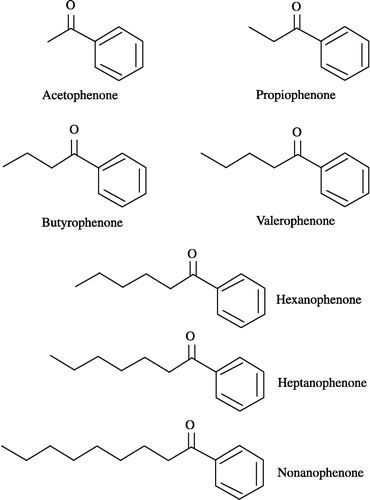
Figure 3 Inhibitory effects of alkyl phenyl ketones on carbonyl reductase activity in pig heart cytosol. 4-BP at a concentration of 500 μM was used as the substrate. The concentration of alkyl phenyl ketones added as inhibitor was 500 μM. Each bar represents the mean ± S.D. of three to six experiments.
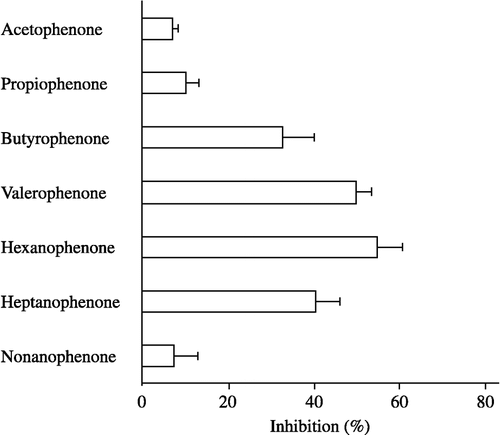
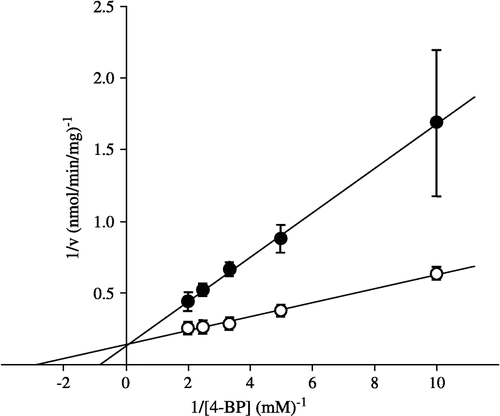
Table I. Kinetic parameters for the reduction of alkyl phenyl ketones in pig heart cytosol.
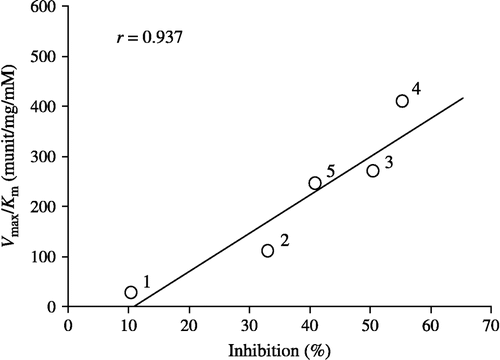
Figure 4 Relationship between Vmax/Km values for the reduction of alkyl phenyl ketones and their inhibitory potencies for carbonyl reductase activity in pig heart cytosol. Plot: 1, propiophenone; 2, butyrophenone; 3, valerophenone; 4, hexanophenone; 5, heptanophenone.