Figures & data
Figure 1 Structures of rhinacanthin–M (1), –N (14), –Q (15) and related naphthoquinone esters (2–13, 16–42).
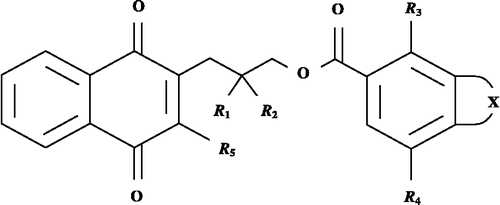
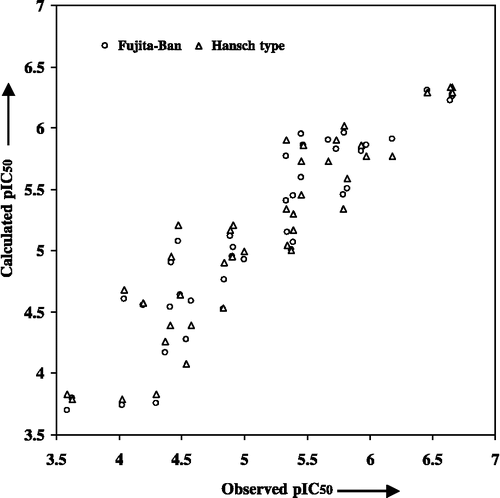
Table I. Observed, calculated and predicted cytotoxicities of rhinacanthins and naphthoquinone esters (Figure 1) against human carcinoma cell lines (KB, HeLa and HepG2).
Table II. QSAR parameters for the substituents of varying positions of the title compounds.
Table III. Fujita-Ban contribution of substituents and parent compound (7) to the cytotoxicity against human carcinoma KB cell lines of the title compounds.
Table IV. Intercorrelation matrixa amongst the predictor variables of Equation (6).