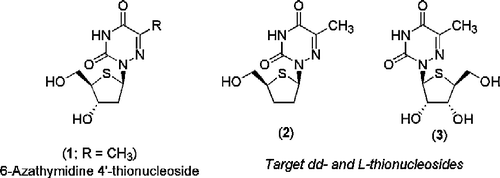
Figures & data
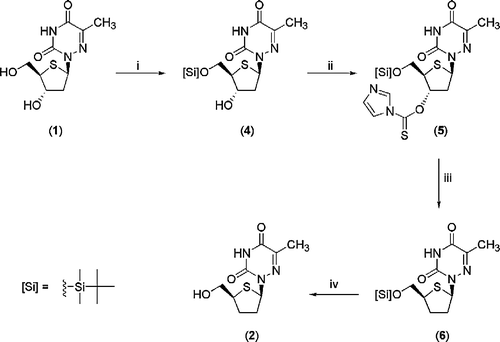
Scheme 1 Reagents and Conditions: (i) TBDMSCl, imidazole, DMF, 3.5 h (ii) thiocarbonyldiimidazole, CH2Cl2, 40°C, 8 h then r.t. 12 h (iii) Bu3SnH, 1,1′-azobis(cyclohexene-carbonitrile), toluene, 100°C, 30 min (iv) Dowex 50W (H+), MeOH, 24 h.
Scheme 2 Reagents and Conditions: (i) 6-Azathymine, BSA, CH3CN, 30 min then 7, TMSOTf, CH3CN, 50°C, 12 h (ii) CH3NH2 (35% aqueous), 50°C, 30 min.
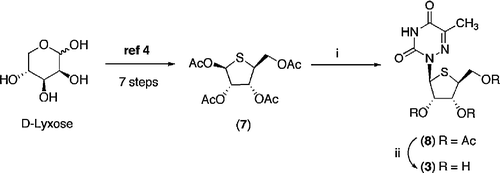
Table I. Cytotoxicity and antiviral activity of novel 4′thionucleosides 2 and 3.
Table II. Thymidine kinase affinity of dideoxynucleoside 2.