Figures & data
Scheme 1 Synthesis of 3-(2-ethylphenyl)-2-substitutedamino-3H-quinazolin-4-ones. Reagents and conditions: (a) DMSO, rt, 30 min; (b) (CH3)2SO4, 5-10°C, 2 h; (c) Methyl anthranilate (3), K2CO3, ethanol reflux for 22 h; (d) 2% alcoholic NaOH, (CH3)2SO4, rt, 1 h; (e) NH2NH2, K2CO3, ethanol reflux for 39 h; (f) (R2R1)CO; CH3COOH reflux, 36 h.
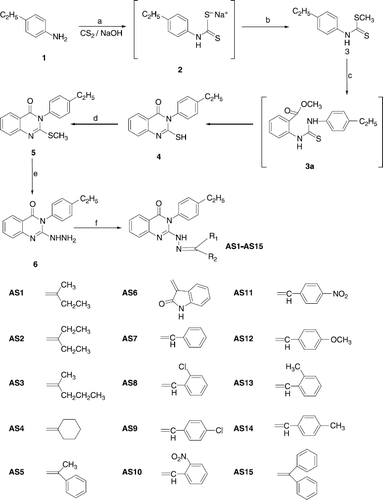
Table I. Analgesic activity of AS1-AS15.
Table II. Anti-inflammatory activity of synthesized compounds (AS1-AS15).
Table III. Evaluation of ulcerogenicity index.