Figures & data
Figure 1. Chemical structure of paroxetine (A), sertraline (B), imipramine (C) and clomipramine (D).
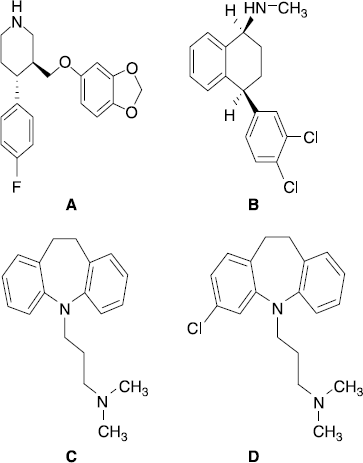
Figure 2. Acetylcholinesterase (AChE) and butrylcholinesterase (BChE) experiments in the presence of 0.5 mM of antidepressants (Clom; clomipramine, Sert; sertraline, Paro; paroxetine and Imip; imipramine). Hydrolysis rates were measured at 412 nm by using 0.4 mM substrate in 1 mL assay solutions with 62 mM phosphate buffer (pH 7.5) and 1 mM DTNB [5,5-dithiobis(2-nitronenzoic acid)]. Enzyme was preincubated for 30 min before 0.4 mM substrate addition. For AChE activity, 0.06 mM ethopropazine (specific inhibitor of BChE) was used to inhibit the activity of BChE in snake venom. All experiments were repeated at least three times and similar results were obtained. For snake venom AChE *P < 0.05 and for human serum BChE *P < 0.038. Significantly different from control.
![Figure 2. Acetylcholinesterase (AChE) and butrylcholinesterase (BChE) experiments in the presence of 0.5 mM of antidepressants (Clom; clomipramine, Sert; sertraline, Paro; paroxetine and Imip; imipramine). Hydrolysis rates were measured at 412 nm by using 0.4 mM substrate in 1 mL assay solutions with 62 mM phosphate buffer (pH 7.5) and 1 mM DTNB [5,5-dithiobis(2-nitronenzoic acid)]. Enzyme was preincubated for 30 min before 0.4 mM substrate addition. For AChE activity, 0.06 mM ethopropazine (specific inhibitor of BChE) was used to inhibit the activity of BChE in snake venom. All experiments were repeated at least three times and similar results were obtained. For snake venom AChE *P < 0.05 and for human serum BChE *P < 0.038. Significantly different from control.](/cms/asset/34ef5041-a603-4477-b995-ffbca0956622/ienz_a_281057_f0002_b.gif)
Figure 3. The graphs show rat brain striatum acetylcholinesterase experiments in the presence of 0.5 mM different antidepressants (Clom; clomipramine, Sert; sertraline, Paro; paroxetine and Imip; imipramine). Hydrolysis rates were measured at 412 nm by using 0.4 mM substrate in 1 mL assay solutions with 62 mM phosphate buffer (pH 7.5), and 1 mM DTNB [5,5-dithiobis(2-nitronenzoic acid)]. Enzyme was preincubated for 30 min before 0.4 mM substrate addition. 0.06 mM ethopropazine (specific inhibitor of BChE) was used to inhibit BChE activity. All experiments were repeated at least three times and similar results were obtained.
![Figure 3. The graphs show rat brain striatum acetylcholinesterase experiments in the presence of 0.5 mM different antidepressants (Clom; clomipramine, Sert; sertraline, Paro; paroxetine and Imip; imipramine). Hydrolysis rates were measured at 412 nm by using 0.4 mM substrate in 1 mL assay solutions with 62 mM phosphate buffer (pH 7.5), and 1 mM DTNB [5,5-dithiobis(2-nitronenzoic acid)]. Enzyme was preincubated for 30 min before 0.4 mM substrate addition. 0.06 mM ethopropazine (specific inhibitor of BChE) was used to inhibit BChE activity. All experiments were repeated at least three times and similar results were obtained.](/cms/asset/ce06af75-422c-4ba3-a95c-eb195f1c5e05/ienz_a_281057_f0003_b.gif)
Figure 4. Kinetic analysis of inhibition of snake venom acetylcholinesterase by Paroxetine (A) Sertraline (B) Imipramine (C) and Clomipramine (D).
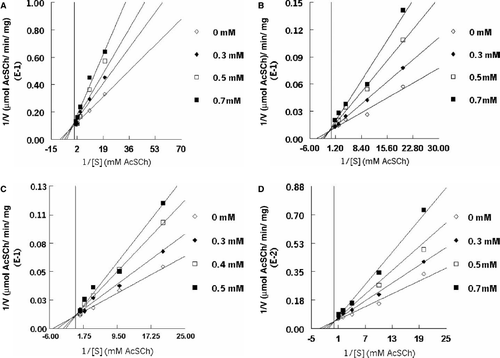
Table I. Ki and IC50 values for venom acetylcholinesterase.
