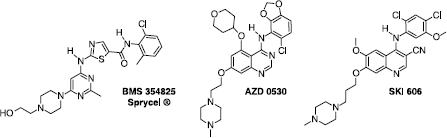
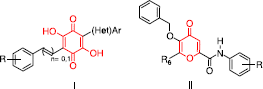Figures & data
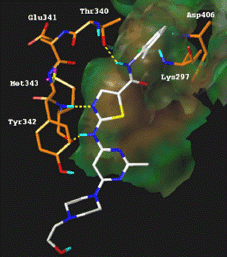
Figure 3 Illustration of a possible binding mode of BMS-354825 in the ATP binding site of active Src kinase.
Scheme 1 Synthesis of 5-benzyloxy-4-oxo-4H-pyran-2-carboxamide and [6-aminocarbonyl-3-benzyloxy-4-oxo-4H-pyran-2-yl]methyl diethyl phosphate derivatives 18-37. Reagents and conditions: (i) DHP, PTSA·H2O, CH2Cl2, rt, 2; (ii) HCHO, NaOH/H2O, rt, 3; (CH3)2CHCHO, NaOH/H2O, rt, 4; HCHO, NaOH/H2O, MeOH, rt, 5; (iii) BnBr, NaOH/H2O, MeOH, Δ, 6-9; (iv) ClP(O)(OC2H5)2, pyridine, DMAP, CH2Cl2, rt, 10; (v) Zn/HCl, H2O, 70°C, 11, 12; (vi) HCl 1 N, Δ, 13; (vii) CrO3, H2SO4, acetone, 0°C or − 20°C, 14-17; (viii) Method A: TBTU, Et3N, toluene/acetonitrile, rt, 18-20; Method B: DEPBT, DIEA, THF, Δ, 21, 22; Method C: CMPI, Et3N, CH2Cl2, Δ, 23-37.
![Scheme 1 Synthesis of 5-benzyloxy-4-oxo-4H-pyran-2-carboxamide and [6-aminocarbonyl-3-benzyloxy-4-oxo-4H-pyran-2-yl]methyl diethyl phosphate derivatives 18-37. Reagents and conditions: (i) DHP, PTSA·H2O, CH2Cl2, rt, 2; (ii) HCHO, NaOH/H2O, rt, 3; (CH3)2CHCHO, NaOH/H2O, rt, 4; HCHO, NaOH/H2O, MeOH, rt, 5; (iii) BnBr, NaOH/H2O, MeOH, Δ, 6-9; (iv) ClP(O)(OC2H5)2, pyridine, DMAP, CH2Cl2, rt, 10; (v) Zn/HCl, H2O, 70°C, 11, 12; (vi) HCl 1 N, Δ, 13; (vii) CrO3, H2SO4, acetone, 0°C or − 20°C, 14-17; (viii) Method A: TBTU, Et3N, toluene/acetonitrile, rt, 18-20; Method B: DEPBT, DIEA, THF, Δ, 21, 22; Method C: CMPI, Et3N, CH2Cl2, Δ, 23-37.](/cms/asset/fb4f753c-0e92-428e-906c-062059c252b9/ienz_a_320696_f0005_b.gif)
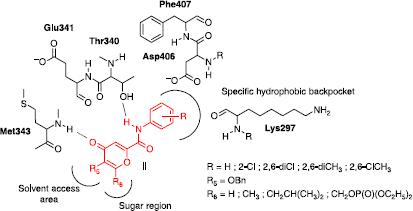
Table I. Amide bond formation.