Figures & data
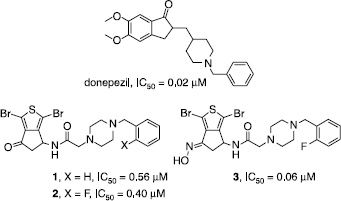
Scheme 1 Synthesis of compounds 1 and 2. Reagents: (i) AcONH4, CH2(COOH)2, EtOH; (ii) TFA2O, Et2O; (iii) Br2, CH2Cl2; (iv) SOCl2; (v) AlCl3, CH2Cl2; (vi) HCl, H2O, EtOH; (vii) TEA, BrCOCH2Br, CH2Cl2; (viii) N-substituted benzylpiperazine, K2CO3, CH2Cl2.
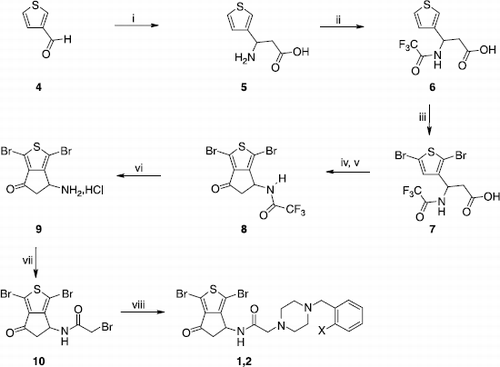
Scheme 2 Synthesis of compounds 3,11-13. Reagents: (i) NH2OH·HCl, AcONa, EtOH, H2O; (ii) NaBH4, MeOH; (iii) DAST, CH2Cl2; (iv) SOCl2, CH2Cl2.
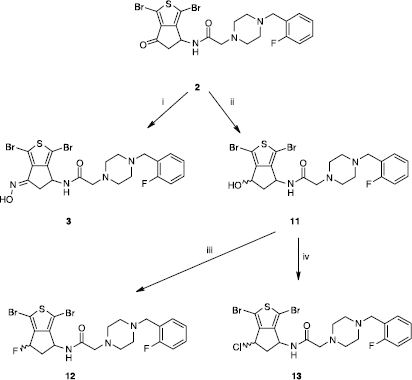
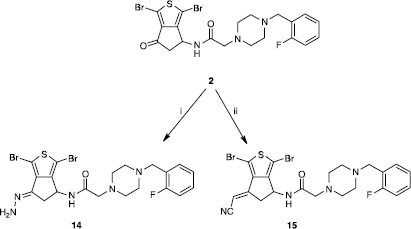
Scheme 3 Synthesis of compounds 14 and 15. Reagents: (i) NH2·NH2·xH20, MeOH; (ii) NCCH2PO(OEt)2, K2CO3, THF.
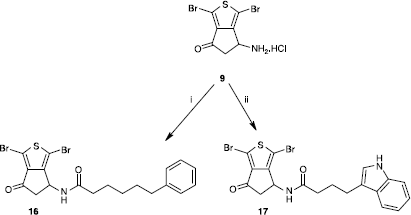
Scheme 4 Synthesis of compounds 16 and 17. Reagents: (i) a- Ph(CH2)5CO2H, TEA, ClCO2Et, CH2Cl2. b- 9, TEA, MeCN; (ii) a- 3-indolebutyric acid, TEA, ClCO2Et, CH2Cl2. b- 9, TEA, MeCN.
Scheme 5 Synthesis of compound 19. Reagents: (i) TEA, BrCO(CH2)3Br, CH2Cl2; (ii) 1,2,3,4-tetrahydroisoquinoline, K2CO3, CH2Cl2.
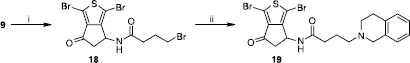
Table I. Anti-AChE activity of compounds 2, 3, 11-15.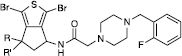 .
.
Table II. Anti-AChE activity of compounds 1, 17, 18, 20.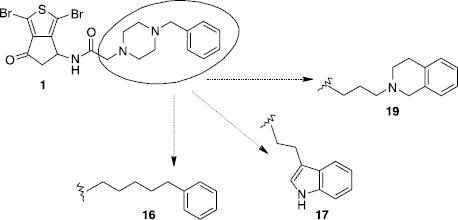 .
.