Figures & data
Scheme 1 Synthesis of indo-3-ylcarboxamides and acetamides 16-30. Reagents and conditions: (a) 1M HCl, EtOH, reflux; (b) NaH, DMF, CH3I or C2H5I, rt; (c) Cs2CO3, CH3CN, BnCl or 4F-BnCl, reflux; (d) 1) 2M NaOH, EtOH, reflux, 2) 2M HCl; (e) POCl3, DMF, 10 °C; (f) NaClO2, NaH2PO4, 2-methyl-2-butene, tBuOH, THF, rt; (g) 2-chloro-1-methylpyridinium iodide, THF, reflux; (h) PPh3, CBrCl3, THF, reflux; (i) phenyldichlorophosphate, CH2Cl2, rt; (j) DCC, THF, rt.
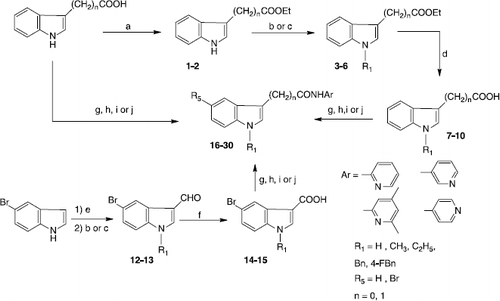
Table I. Compounds tested on hyaluronidase modulating activity at pH 7 and pH 3.5.
Table II. Hyaluronidase activity in the presence of compounds 16 – 30 in a concentration of 50 μM at pH 7 (stains-all assay) and pH 3.5 (Morgan-Elson assay).
Table III. Inhibitory effect of compounds 22, 23 and 24 (50 μM) on hyaluronidase activity at pH 7 (Morgan-Elson assay).
Figure 1 Change of the protonation state of compound 24 (N-(4,6-Dimethylpyridin-2yl)-(1-ethylindole-3-yl)acetamide) from pH 7 to pH 3.5.
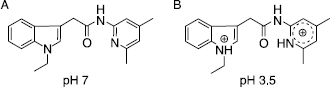
Figure 2 Concentration dependent activation of BTH by compounds 22 (▴), 23 (▪) and 24 (♦) in the Morgan-Elson assay at pH 3.5.
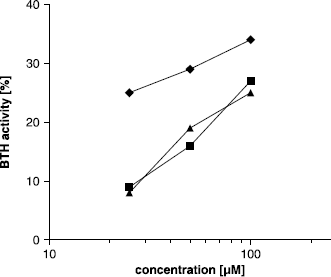
Figure 3 Activating effect of BSA on BTH activity measured by the stains-all assay at pH 7. Activity without BSA was set to 100%.
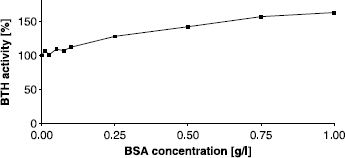
Figure 4 Activating effect of BSA on BTH activity at pH 3.5 (Morgan-Elson assay) without additional activator (▴) and after addition of compound 24 in a concentration of 100 μM (▪). BTH activity with 44 mg/L BSA, as used in our standard protocol of the Morgan-Elson assay, was set to 100%.
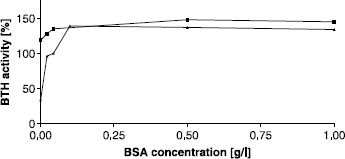
Figure 5 Interactions between the protonated form of compound 24 (ball-and-stick model) and HA (stick model) mediated by saltbridges. Only hydrogens in hydroxyl groups or connected to nitrogen are shown. Building of the compound and visualization of both molecules were performed using the program SYBYL from Tripos (www.tripos.com). HA8 molecule was taken from the RCSB protein data bank (PDB accession code: 2BVK) [Citation46].
![Figure 5 Interactions between the protonated form of compound 24 (ball-and-stick model) and HA (stick model) mediated by saltbridges. Only hydrogens in hydroxyl groups or connected to nitrogen are shown. Building of the compound and visualization of both molecules were performed using the program SYBYL from Tripos (www.tripos.com). HA8 molecule was taken from the RCSB protein data bank (PDB accession code: 2BVK) [Citation46].](/cms/asset/cf3eb757-62f2-41e4-94e9-decfd315715b/ienz_a_320982_f0006_b.gif)