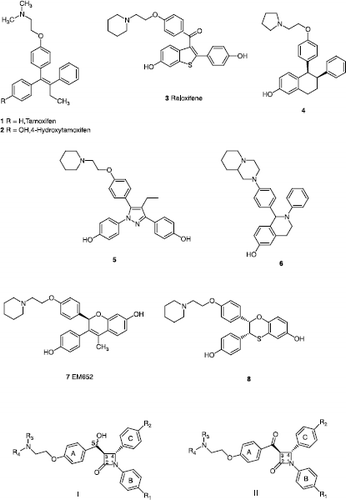
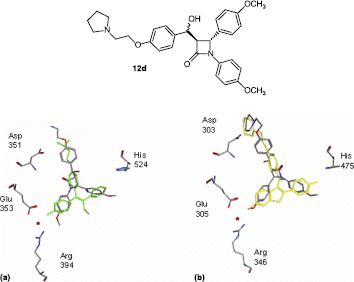
Figures & data
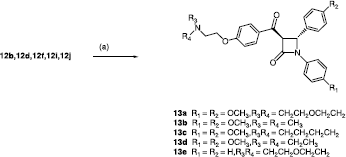
Scheme 1 Synthesis of β-lactams 12a–u. Scheme Reagents: (a) CH3CH2OCOCH2Br, Zn, TMCS, Benzene, reflux, 6h. (b) aldehyde/ketone, LDA, THF, 78°C, 30min. (c) R3R4N-CH2CH2Cl, Acetone, reflux, 2 h.
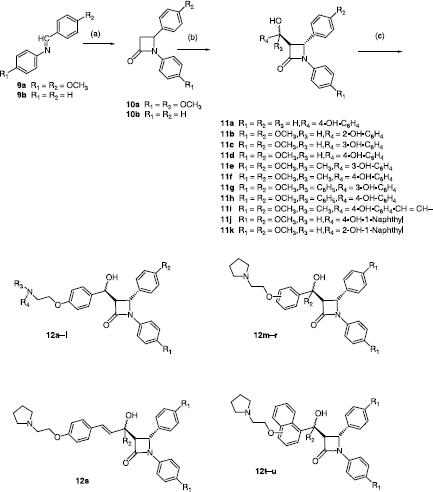
Table I. Antiproliferative and cytotoxic effects of compounds 12a–l in MCF-7 cells.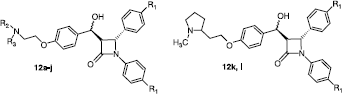 .
.
Table II. Antiproliferative and cytotoxic effects of compounds 13a–e containing carbonyl at C-5 in MCF-7 cells.
Table III. Antiproliferative and cytotoxic effects of compounds 12m–u with modifications at the C-5 position in MCF-7 cells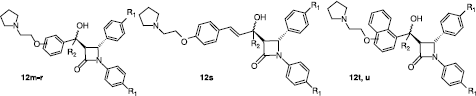 .
.
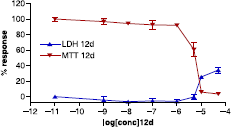
Figure 2 Compound 12u inhibited proliferation and induced cytotoxicity of MCF-7 cells. Antiproliferative and cytotoxic activity of compounds 12u on oestrogen sensitive MCF-7 breast cancer cells, IC50 = 5.51 μM. The optical density values are given as a ratio of the treated cells and control cells × 100% and are means of at least 9 replicates. The absence of error bars indicates that the error was smaller than the size of the symbol.