Figures & data
Figure 2. MT activation in the catecholase reactions with MeBACat as substrate in 10 mM PBS, 20 °C and pH 6.8, in the presence of pyruvic acid (○), acrylic acid (♦), propanoic acid (▵), 2-oxo-butanoic acid (□) and 2-oxo-octanoic acid (◊).
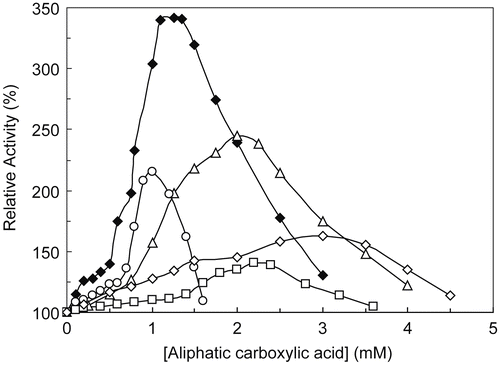
Table 1. Activation and inhibition parameters in catecholase and cresolase reactions
Figure 3. (a) Double reciprocal Lineweaver-Burk plots of MT kinetic assays for cresolase reactions with MePAPh in 10 mM PBS, 20 °C and pH 6.8, in the presence of different fixed concentrations of propanoic acid: 0 (♦), 0.5 (□), 1.0 (▴), 1.25 (×) and 1.5 mM (*). (b) The secondary plot of reciprocal apparent maximum velocity (1/ V′max) versus the concentration of inhibitor [I], where the abscissa-intercept is −Ki.
![Figure 3. (a) Double reciprocal Lineweaver-Burk plots of MT kinetic assays for cresolase reactions with MePAPh in 10 mM PBS, 20 °C and pH 6.8, in the presence of different fixed concentrations of propanoic acid: 0 (♦), 0.5 (□), 1.0 (▴), 1.25 (×) and 1.5 mM (*). (b) The secondary plot of reciprocal apparent maximum velocity (1/ V′max) versus the concentration of inhibitor [I], where the abscissa-intercept is −Ki.](/cms/asset/979e81b3-a469-4205-aaca-53a5f79b9541/ienz_a_363435_f0003_b.gif)
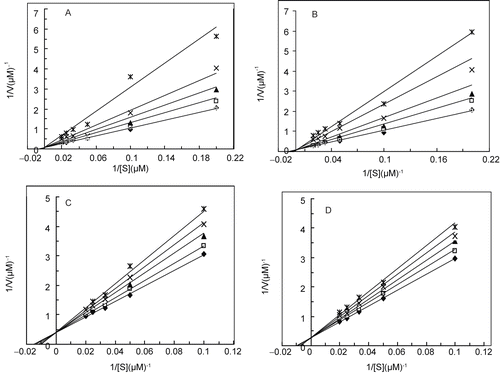
Figure 4. Double reciprocal Lineweaver-Burk plots of MT kinetic assays for cresolase reactions with Me-PAPh in 10 mM PBS, 20 °C and pH 6.8, in the presence of different fixed concentrations of 0 (♦), 0.1 (□), 0.2 (▴), 0.4 (×) and 0.75 mM (*) for pyruvic acid (a); 0 (♦), 0.2 (□), 0.4 (▴), 0.8 (×) and 1.2 mM (*) for acrylic acid (b); 0 (♦), 0.4 (□), 1 (▴), 1.5 (×) and 2.5 mM (*) for 2-oxo-butanoic acid (c) and 0 (♦), 0.5 (□), 1 (▴), 1.5 (×) and 2 mM (*) for 2-oxo-octanoic acid (d).

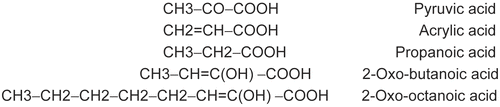
![Figure 5. The secondary plots of the slopes for the lines in Figure 4 (a–d) against the concentration of inhibitor, [I], gives a straight line with an abscissa intercept of inhibition constant (−Ki) for pyruvic acid (a), acrylic acid (b), 2-oxo-butanoic acid (c) and 2-oxo-octanoic acid (d).](/cms/asset/cda9901a-b43c-4150-833f-3ada2dc5237f/ienz_a_363435_f0005_b.gif)