Figures & data
Figure 1. (a) Inactivation progress curves as a dependence of urease residual activity vs incubation time at pH 7.8, for different DCNQ concentrations. (b) Dependence of urease residual activity vs incubation time in semilogarithmic system. DCNQ concentration is given numerically.
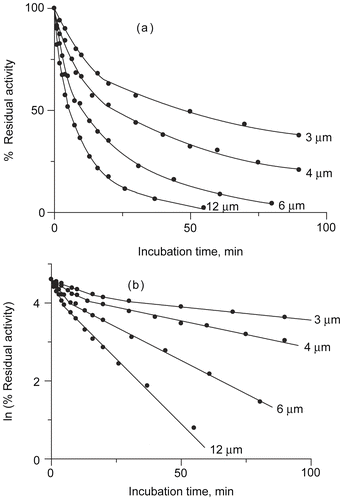
Table 1. Kinetic constants of urease inactivation by DCNQ: the rate constants of the fast (kf) and slow phase (ks) and the slow phase contribution parameter a.
Figure 2. Urease residual activity as a function of DCNQ concentration for a 20 minute and 60 minute incubation.
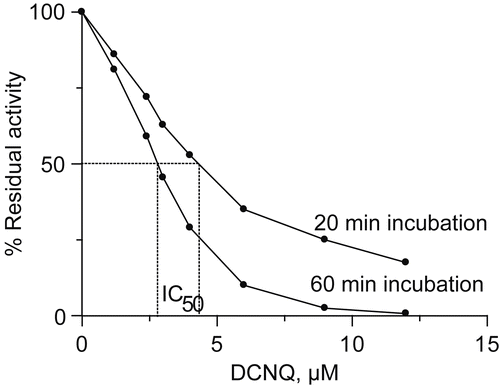
Figure 3. Thiol influence on urease inactivation by DCNQ relative to the control activity. The percent of the enzyme activity in the presence of DCNQ without the thiol is given for comparison. Concentration of the thiols: L-cysteine (L-cys), glutathione (GSH), dithiothreitol (DTT) and DCNQ in the incubation mixture was equal to 0.5 mM and 8 μM, respectively. Enzyme activity was determined after a 10 and 30 minutes incubation.
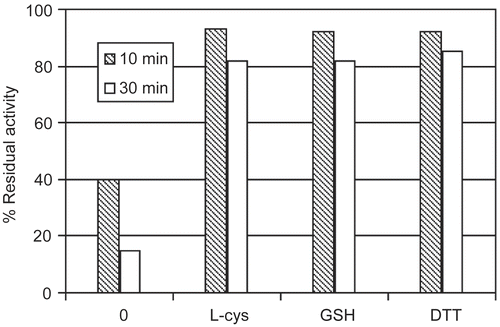
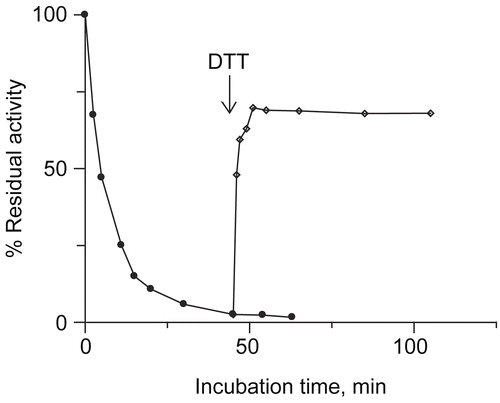
Figure 4. Catalase influence on urease inactivation by DCNQ. DCNQ concentration was equal to 5.5 and 11 μM.
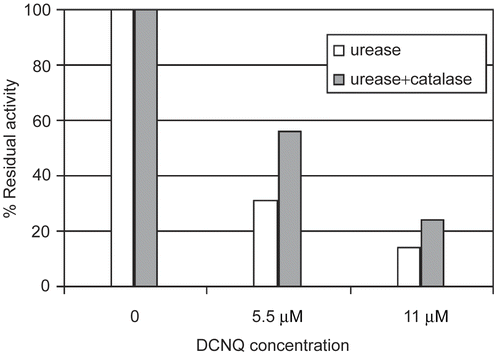
Table 2. Estimation of hydrogen peroxide production by 7 μM DCNQ in 20 mM phosphate buffer pH 7.8, after a 60 min incubation.