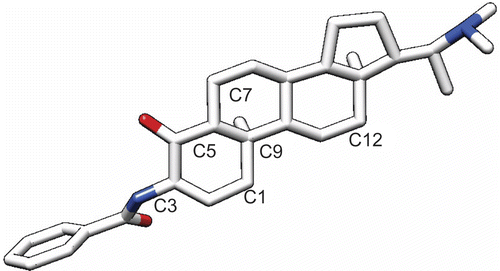
Figures & data
Figure 2. Axillaridine–A located at the aromatic gorge of AChE (PDB code: 1B41) after 2-ns MD simulation. Only the active site amino acids are labeled for the sake of clarity.
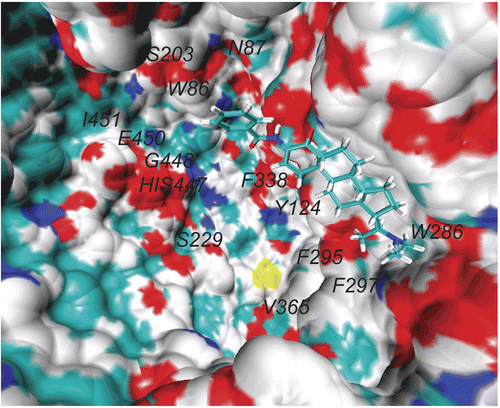
Figure 3. Important amino acid residues within 5.0 å at the active site of AChE after 2-ns MD simulation. For detail refer to the text.
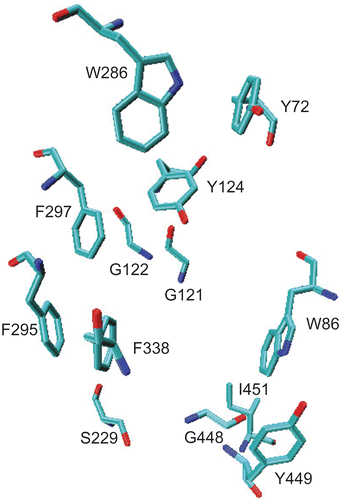
Figure 4. Size (Å) of the aromatic gorge between Glu81/Try286 (Upper) and Phe338/Trp86 (Lower) during 2-ns AChE-Axillaridine-A simulation.
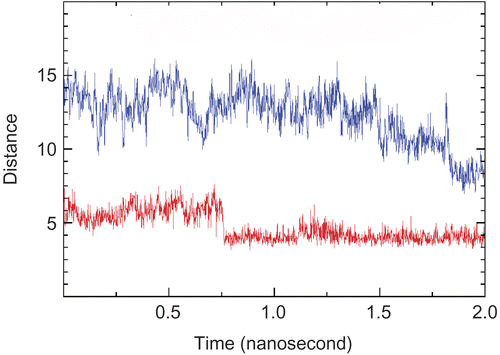
Figure 5. Stable π–π interactions between Trp286/Tyr72 (Lower) and the aromatic ring of Axillaridine–A/Tyr124 (Upper) as a function of time in the AChE-Axillaridine-A simulation.
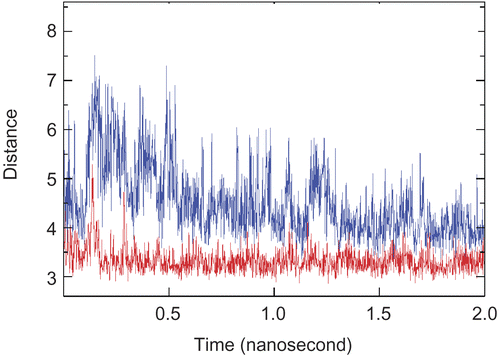
Figure 6. π-π Interaction between the aromatic ring of Axillaridine-A (shown in gray color) and that of the Tyr124 residue (shown in orange color).
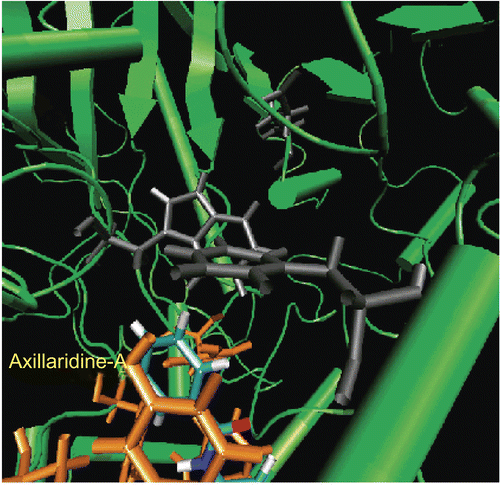
Table 1. Summary of the average distances between heavy atoms (Å) for important stable hydrogen-bonding interactions between active site residues.