Figures & data
Figure 2. Dose-dependent inhibition of compound 1 on PTP1B enzyme activity. The means with S.D. of three independent determinations are shown.
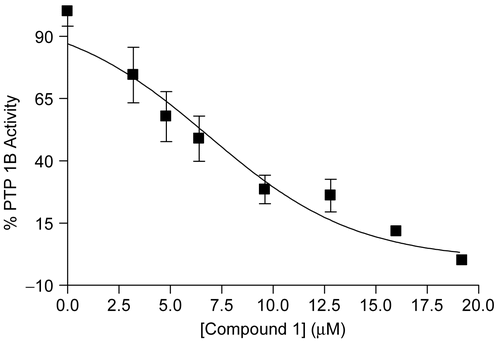
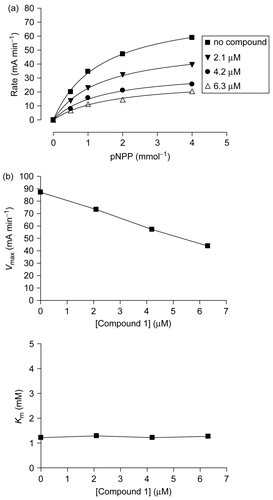
Figure 3. Non-competitive inhibitor of PTP1B. (a) Substrate titration reveals that compound 1 is a classical non-competitive inhibitor that inhibits substrate catalysis (Vmax), but not substrate binding (constant Km). (b) Plots of Vmax and Km as a function of concentration of compound 1. Values were derived from nonlinear regression analysis in (a).