Figures & data
Figure 1. Process curves for the inhibition of monophenolase of mushroom tyrosinase by tiliroside. Tyrosine was a substrate. The reaction was done in 50 mM Na2HPO4-NaH2PO4 buffer, pH 6.6, at 30 °C in the presence of different concentrations of tiliroside for curves 1–6 were 0, 0.021, 0.042, 0.084, 0.168 and 0.337 mM, respectively. The final concentration of mushroom tyrosinase was 14.934 μg/mL.
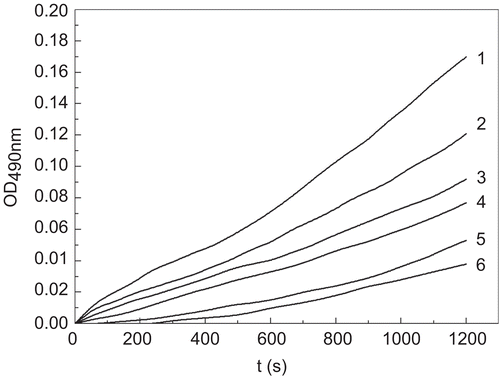
Figure 3. Lineweaver-Burk plots for inhibition of tiliroside on the oxidation of tyrosine by mushroom tyrosinase. Tyrosine was a substrate. The reaction was done in 50 mM Na2HPO4-NaH2PO4 buffer, pH 6.6 and at 30 °C in the presence of different concentrations of tiliroside for curves 1–5 was 0, 0.042, 0.084, 0.168 and 0.337 mM, respectively. The final concentration of mushroom tyrosinase was 14.934 μg/mL. The inset was the secondary plot of the slope versus concentration of inhibitor (tiliroside).
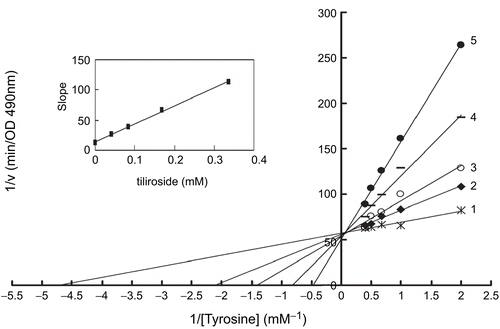
Figure 4. Lineweaver-Burk plots for inhibition of tiliroside on the oxidation of L-DOPA by mushroom tyrosinase. L-DOPA was a substrate. The reaction was done in 50 mM Na2HPO4-NaH2PO4 buffer, pH 6.6, at 30 °C in the presence of different concentrations of tiliroside for curves 1–5 was 0, 0.042, 0.084, 0.168 and 0.337 mM, respectively. The final concentration of mushroom tyrosinase was 3.000 μg/mL. The inset was the secondary plot of the slope versus concentration of inhibitor (tiliroside).
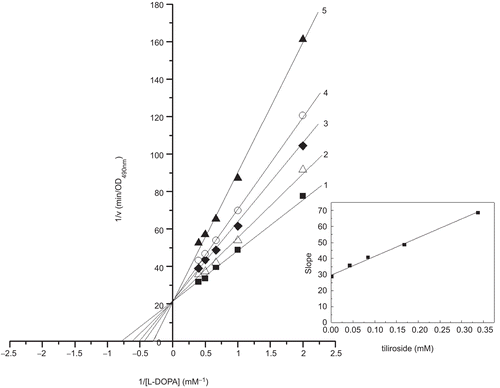
Table 1. The cell viability of tiliroside and arbutin on B16 cells (Mean ± SD, %).
Table 2. The tyrosinase- and melanin-reducing activities of tiliroside on B16 cells.