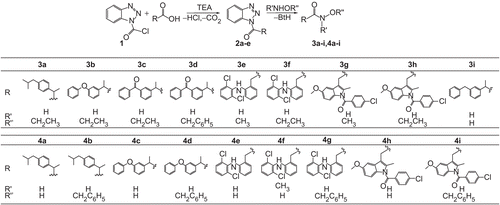
Figures & data
Table 1. Reaction conditions and analytical data for compounds 3a–i.
Table 2. Spectroscopic data and atom enumeration for compounds 3a–i.
Table 3. Antimicrobial activity of NSAID hydroxamic acid derivatives 3 and 4 determined by hole-plate diffusion method.
Table 4. Minimal inhibitory (MIC) and minimal microbicidal concentrations (MMcC) of NSAID hydroxamic acid derivatives 3 and 4 determined by microdilution broth methoda.
Figure 2. Dose-dependent inhibition of jack bean urease by NSAID hydroxamic acid derivatives: 3i (▴), 4a (♦), 4c (▪), 4e (*), 4h (•).
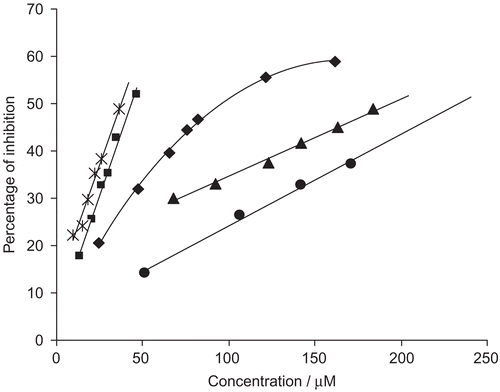
Table 5. Interaction with DPPH, in vitro inhibition of soybean lipoxygenase (LO), lipid peroxidation (LP), and theoretically calculated Clog P values.