Figures & data
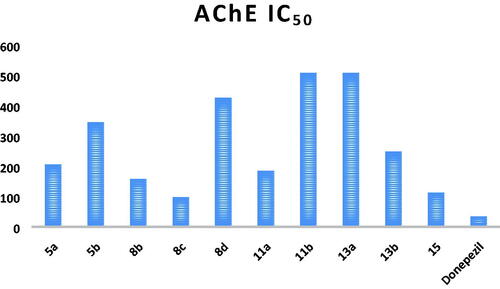
Table 1. IC50 values of the tested compounds and the reference drug donepezil against AChE enzyme.
Table 2. BuChE IC50, selectivity index, MMP-2 IC50, and self-induced Aβ1–42 aggregation IC50.
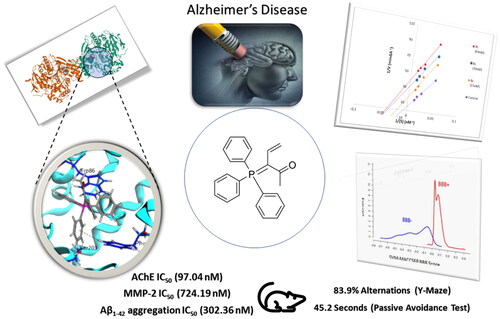
Figure 5. (a) Kinetic study on the mechanism of AChE inhibition by compound 8c, Overlaid Lineweaver-Burk reciprocal plots of AChE initial velocity at increasing substrate concentration (15–60 nM) in the absence and in the presence of different concentrations of 8c. (b) Dixon plot of compound 8c showing the Ki value as negative intercept on the X-axis.
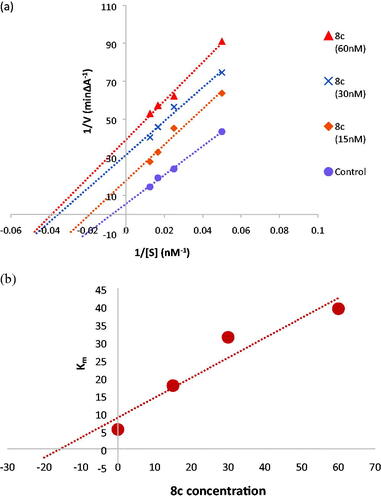
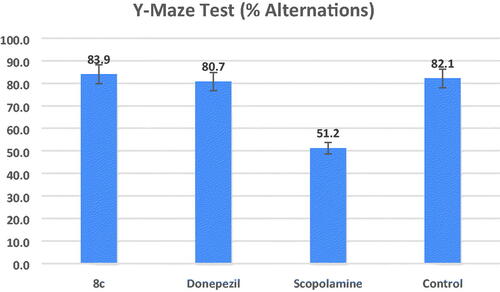
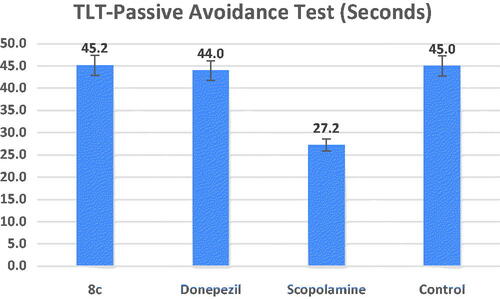
Figure 7. Effects of compound 8c on the % of spontaneous alternations in the Y-maze test compared to the reference drug donepezil, the data shown are mean ± SD (n = 5). #p < 0.01 vs. control group.