Figures & data
Figure 1. The role of tumor-associated neutrophils in PDAC. PDAC cells secrete chemokines (such as IL-8, CXCR1, CXCR2, CXCR4, etc.) and cytokines (such as G-CSF, GM-CSF, etc.), which recruit neutrophils from the circulation system and direct them to the tumor microenvironment, where they polarized into pro-tumor TAN under the influence of TGF-β produced by tumor cells and other immune cells. These TANs not only secrete MMP-9 and VEGF to stimulate angiogenesis, but also induce tissue damage and tumorigenesis of normal ductal epithelial cells by generating ROS and proteases or inducing NETs. In addition, such TANs also express PD-L1, which interacts with PD-1 on T cells to facilitate checkpoint mediated immune escape. CXCR1, CXC motif chemokine receptor 1; CXCR2, CXC motif chemokine receptor 2; CXCR4, CXC motif chemokine receptor 4; G-CSF, granulocyte colony stimulating factor; GM-CSF, granulocyte-macrophage colony stimulating factor; IL-8, interleukin 8; IL-17, interleukin 17; MMP-9, matrix metalloproteinase-9; NETs, neutrophil extracellular traps; PanIN, pancreatic intraepithelial neoplasia; PD-1, programmed cell death; PD-L1, programmed death-ligand 1; PDAC, pancreatic ductal adenocarcinoma; ROS, reactive oxygen species; TAN, tumor-associated neutrophil; TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor.
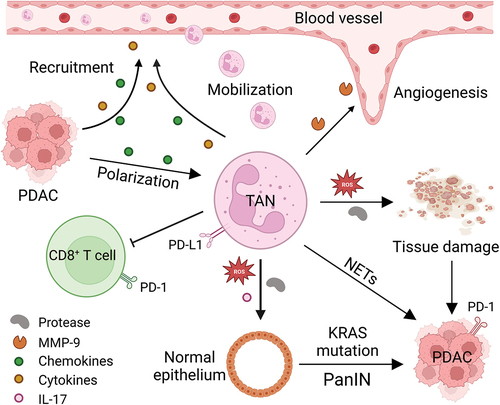
Figure 2. Mechanisms of NETosis. Exogenous microorganisms (such as bacteria, fungi, viruses, etc.) and immune complexes activate neutrophils by stimulating receptors on the cell membrane, such as TLR2, C3R, IL-1, CD18. Activated neutrophils induce the release of Ca2+ from the endoplasmic reticulum. Intracellular Ca2+ efflux activates protein kinase signaling to stimulate NADPH oxidase to produce ROS, which trigger a MPO pathway. MPO-mediated oxidative activation of NE is required for NE to degrade the actin cytoskeleton in the cytoplasm. MPO and NE subsequently translocate to the nucleus, causing nuclear membrane disruption and chromatin decondensation. The generated NETs are directly released into the cytoplasm, and the rupture of the plasma membrane leads to the release of NETs to the extracellular space and neutrophil death. C3, complement 3; C3R, complement 3 receptor; IL1, interleukin 1; LPS, lipopolysaccharide; MPO, myeloperoxidase; NADPH, nicotinamide adenine dinucleotide phosphate; NE, neutrophil elastase; NETs, neutrophil extracellular traps; ROS, reactive oxygen species; TLR-2, toll-like receptor 2; TNF-α, tumor necrosis factor-α.
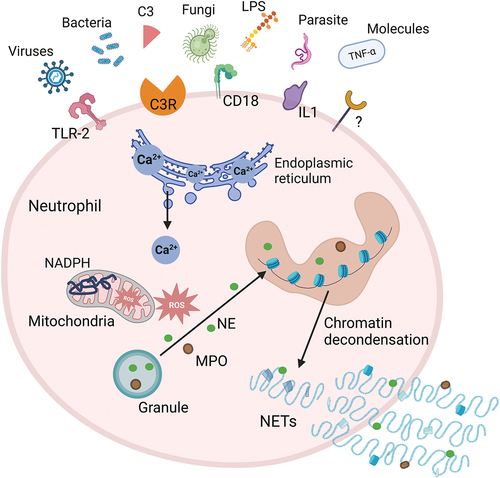
Figure 3. Neutrophil reprogramming and potential targets. The metabolic mode of neutrophils in the circulation is mainly glycolysis. They can be recruited into the TME by chemokines (such as IL-8, CXCR1, CXCR2, CXCR4), where the low glucose and high lipid environment induces a metabolic reprogramming of neutrophils. The high lipid content in the TME, the upregulation of tumor-derived cytokines (G-CSF and GM-CSF), and the upregulation of lipid transport receptors mediated by STAT3 or STAT5 enhance the uptake of exogenous fatty acids by TANs, resulting in fatty acid oxidation that is immunosuppressive. In addition, there are two subtypes of neutrophils within the TME, the anti-tumor N1 type and the pro-tumor N2 type, and these two different phenotypes of TANs can be converted to each other. Blocking the PD-L1 signal in TANs with immune checkpoint inhibitors, inhibiting the formation and/or structure of NETs, inhibiting the angiogenesis derived from TANs, and regulating the recruitment and polarization of neutrophils in the TME are potential targets for reprogramming TANs in tumors. CXCR1, CXC motif chemokine receptor 1; CXCR2, CXC motif chemokine receptor 2; CXCR4, CXC motif chemokine receptor 4; G-CSF, granulocyte colony stimulating factor; GM-CSF, granulocyte-macrophage colony stimulating factor; IL-6, interleukin 6; IL-8, interleukin 8; JAML, junction adhesion molecule-like protein; LOX-1, lectin-like oxidized low-density lipoprotein (LDL) receptor-1; MMP-9, matrix metalloproteinase-9; NADPH, nicotinamide adenine dinucleotide phosphate; NETs, neutrophil extracellular traps; NO, nitric oxide; PD-1, programmed cell death; PD-L1, programmed death-ligand 1; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
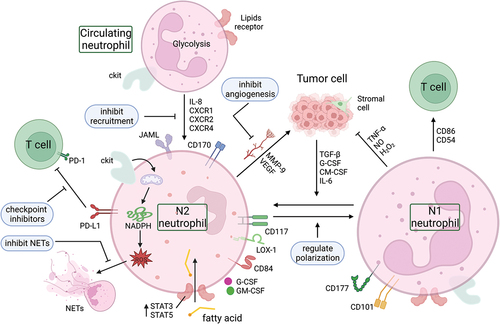
Table 1. Clinical trials targeting neutrophils in treating PDAC.
