Figures & data
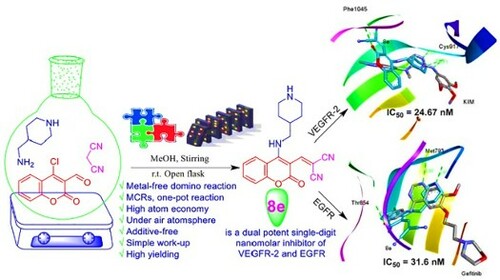
Table 1. Optimization of domino amination-Knoevenagel condensation reaction to form 4aTable Footnotea.
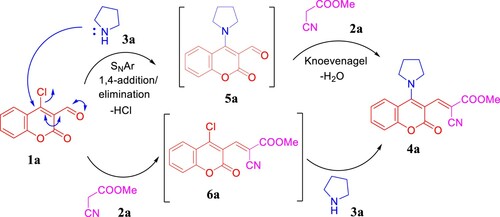
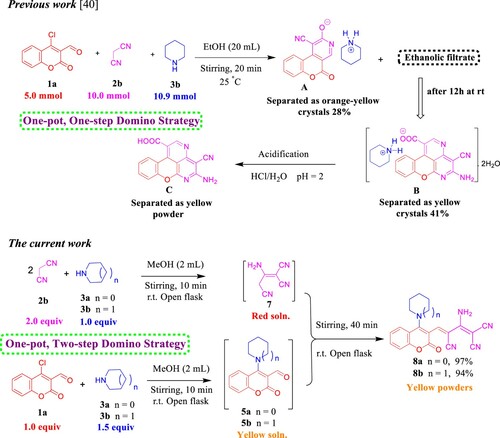
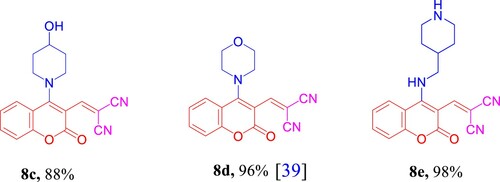
Scheme 1. Scope of various cyclic secondary amines for the synthesis of 4a–d. Reagents and conditions: 1a (1.0 mmol, 1.0 equiv), 2a (1.0 mmol, 1.0 equiv), and 3a–d (2.5 mmol, 2.5 equiv) in MeOH (2 mL) using open flask at room temperature under stirring conditions.
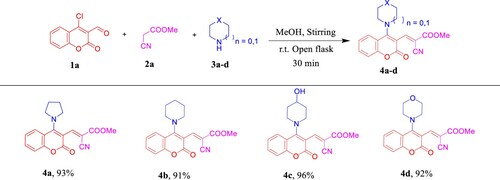
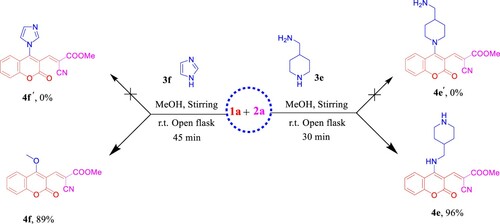
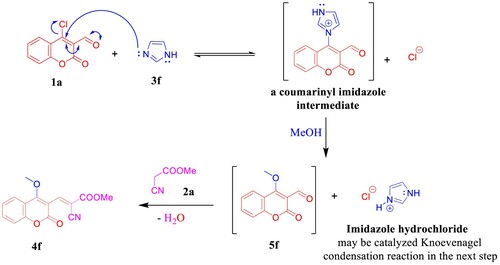
Scheme 6. Previous work by Yang and Vyasamudri groups as well as scope of various cyclic secondary amines for the synthesis of 5a,d–f and 9a,d–f. Reagents and conditions: 1a,c (1.0 mmol, 1.0 equiv), 3a,d–f (1.0 mmol, 1.0 equiv), and Et3N (1 mmol, 1 equiv) in MeOH (2 mL) using open flask at room temperature under stirring conditions, R = CHO, 15 min, R = NO2, 5 min.
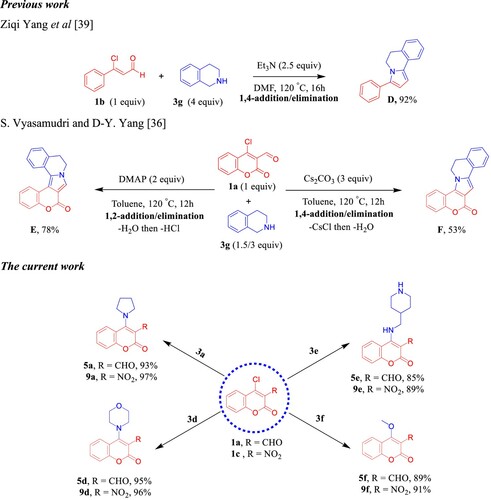
Figure 2. Sigmoidal dose–response against KB-3-1 cell line; (A) 4e, (B) 5e, (C) 8e, (D) 9d, and (E) graph show the IC50 values of the tested compounds 4e, 5e, 8e, 9d, and (+)-griseofulvin as a positive control.
Table 2. IC50 (μM) of all the synthesized compounds against KB-3-1(cervix), A549 (non-small cell lung), PC3 (prostate) human cancer cell lines.
Figure 3. (A) % Viability of A549 (non-small lung) and PC3 (prostate) human cancer cell lines (after 72 h) at 80 µM concentration of compounds 4a–f, 5a, 5d–f, 8a–e, 9a, and 9d–e. (B) Dose–response of compounds 4e, 8e, and 9d against A549 (non-small cell lung) human cancer cell line (72 h). (C) Dose–response of compounds 4d, 4e, 8d, 8e, and 9d against PC3 (prostate) human cancer cell line (72 h).
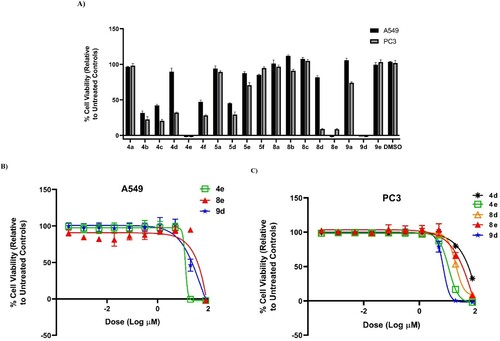
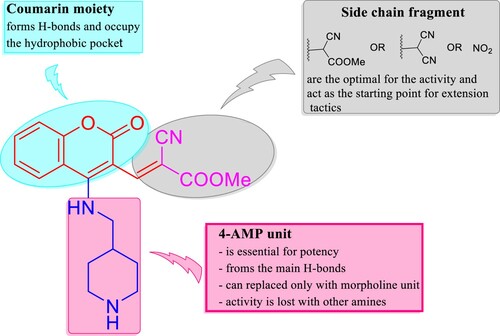
Figure 4. IC50 of compounds 4e, 5e, 8e, and 9d as inhibitors to the VEGFR-2 and EGFR human kinases. Sorafenib is the standard inhibitor to VEGFR-2 enzyme, while erlotinib is the equivalent in the case of the EGFR.
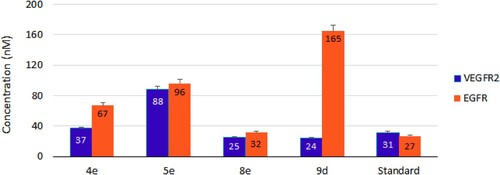
Table 3. VEGFR-2 and EGFR inhibitory activities by compounds 4e, 5e, 8e, and 9d with their fold inactivation relative to the standard.
Table 4. Physicochemical properties, drug-likeness, and medicinal chemistry parameters of compounds 4e, 5e, 8d,e, and 9d.
Figure 5. Ligand-based target prediction by SwissTarget-Prediction web tool; (A) 4e, (B) 5e, (C) 8d, (D) 8e, and (E) 9d.
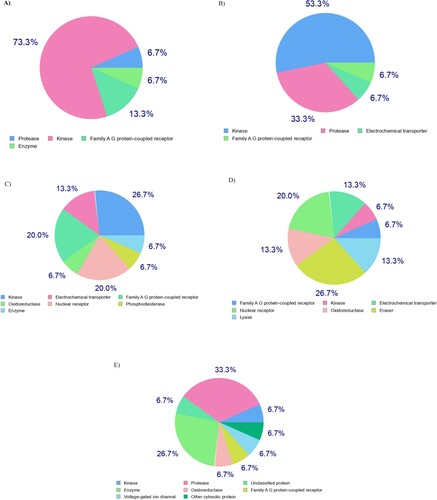
Figure 6. (A, C, E and G) Three-dimensional docking poses of 4e, 8e, 9d, and sorafenib (cyan), respectively, with KIM (grey) within the binding site of VEGFR-2 (PDB ID: 3CJG). (B, D, F and H) Three-dimensional binding modes of 4e, 8e, 9d, and erlotinib (cyan), respectively, with gefitinib (grey) within the active site of EGFR (PDB ID: 4WKQ). H-bonds are denoted by dashed lines in green. All pictures were prepared with Discovery Studio Visualizer Client 2020, and are simple for clarity of presentation.
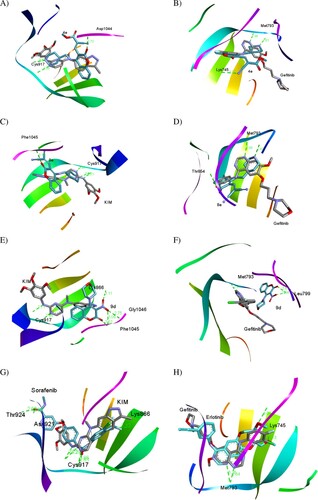
Table 5. Binding fitness and no. of H-bonds of 4e, 8e, and 9d to VEGFR-2 and EGFR in comparison with KIM, sorafenib, gefitinib, and erlotinib as the reference drugs.
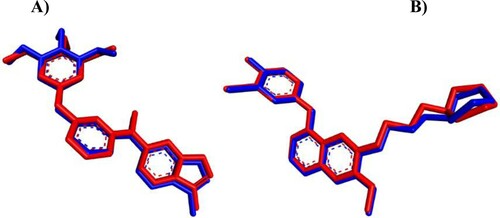
Figure 8. iGEMDOCK validation. (A) Crystal KIM (red) and the docked one (blue) display similar binding orientation in the binding pocket of VEGFR-2 with RMSD 0.4355 Å. (B) Crystal gefitinib (red) with the docked one (blue) are superimposed in EGFR binding cavity with RMSD 0.3462 Å.