Figures & data
Figure 1. Construction of CLL1:TRAIL. (A) Vector map of scFvCLL1:TRAIL, designated CLL1:TRAIL. (B) Amino acid sequence of CLL1:TRAIL. (C) Schematic diagram of CLL1:TRAIL components and active CLL1:TRAIL trimer. (D) Gel stained with coomassie blue of reduced CLL1:TRAIL (β-mercaptoethanol and sample boiling) and corresponding immunoblot (IB) stained with anti-HA-HRP, as well as immunoblot analysis of CLL1:TRAIL (anti-HA-HRP) under nonreducing conditions without sample boiling. Size exclusion FPLC was performed, after which all collected fractions were tested for their potential to induce cytotoxicity in CLL1+ U937 cells. Apoptosis was determined by Annexin-V staining.
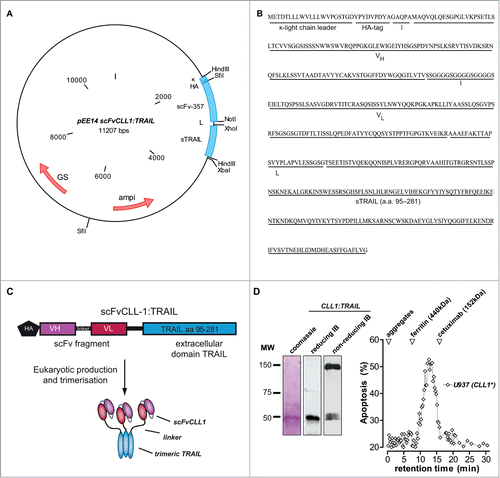
Figure 2. CLL1-resticted binding and apoptotic activity of CLL1:TRAIL. (A) Binding analysis of CLL1:TRAIL to Ramos (CLL1 negative) and Ramos.CLL1 (CLL1 positive) cells, with and without blocking with αCLL1:Fc using flow cytometry. (B) Target-restricted induction of apoptosis by increasing doses of CLL1:TRAIL was analyzed using Ramos and Ramos.CLL1. Apoptosis was analyzed using flow cytometric Annexin-V staining. (C) U937 (CLL1 positive) cells were treated with CLL1:TRAIL (250 ng/ml) in the presence or absence of αCLL1:Fc (2.5 μg/ml) or TRAIL neutralizing mAb2E5 (1 μg/ml) and apoptosis was determined using flow cytometric analysis of DioC6 staining. (D) CLL1:TRAIL was stored for up to 7 d in 5% FCS at −20°C, 4°C, and 37°C, after which CLL1-restricted activity was analyzed on the Ramos.CLL-1/Ramos cell line pair. Apoptosis was determined by Annexin-V staining. All graphs represent mean+SD. * p < 0.05, **p < 0.01, *** p < 0.001.
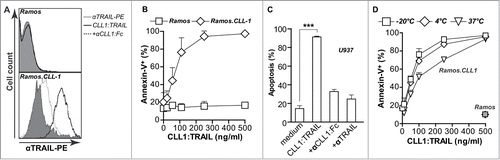
Figure 3 (See previous page). CLL1:TRAIL selectively binds to and augments tumoricidal activity of granulocytes. (A) Flow cytometric analysis of CLL1:TRAIL binding to the surface of granulocytes, with and without αCLL1:Fc. (B) Within the granulocyte (CD16 positive) and monocyte (CD14 positive) population, the binding of CLL1:TRAIL to their cell surface was analyzed. (C) DLD-1 colon carcinoma cells (Target: T) were incubated with increasing amounts of leukocytes (Effector: E) (E:T ratio) in the presence or absence of CLL1:TRAIL (200 ng/ml) or MCSP:TRAIL (200 ng/ml) for 24 h, after which cell viability was assessed by MTS assay. (D) DLD-1 colon carcinoma cells were incubated with increasing concentrations of CLL1:TRAIL in the presence or absence of leukocytes (E:T = 5:1) and cell viability was assessed. CLL1 restricted activity of the fusion protein was evaluated by co-incubation with competing αCLL1:Fc minibody. (E) TRAIL- and caspase-dependent apoptosis was evaluated by co-incubating CLL1:TRAIL (200 ng/ml) and leukocytes (E:T = 5:1) with anti-TRAIL mab2E5 (1 μg/ml), and caspase-8 inhibitor zIETD-fmk (20 μM). (F) As in (C), but cytotoxicity was analyzed using LDH-release assay (G) DLD-1 colon carcinoma cells were incubated with CLL1:TRAIL (500 ng/ml) in co-cultures with increasing amounts of leukocytes or isolated granulocytes. Cell viability was determined using MTS assay. (H) Apoptosis induction of leukocytes (E:T = 10:1) and CLL1:TRAIL (200 ng/ml) was determined on DLD-1 3D-spheroids by Annexin-V staining on trypsin dissociated DLD-1 cells. All graphs represent mean+SD. * p < 0.05, **p < 0.01, *** p < 0.001, n.s. not significant.
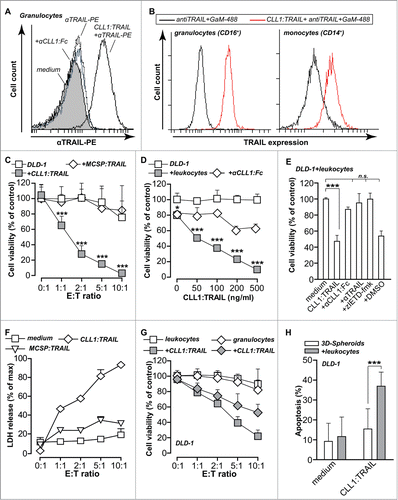
Figure 4. CLL1:TRAIL has no toxicity toward normal cells. (A) The effect of CLL1:TRAIL (500 ng/ml) on CLL-1 positive leukocytes was determined by assessing apoptosis (Annexin-V) by flow cytometry in CD16-positive granulocytes and CD14-positive monocytes, after 16 h treatment (using anti-CD14-APC, anti-CD16-PE-Dy647). Of note, leukocytes were stimulated with 10 ng/ml IFNγ to reduce spontaneous apoptosis which normally occurs in granulocytes. (B) Primary normal human cells were treated with CLL1:TRAIL (700 ng/ml) and apoptosis was measured by DioC6 assays. (C) As in G, in the presence of leukocytes (E:T = 10:1). n.d.: not determined
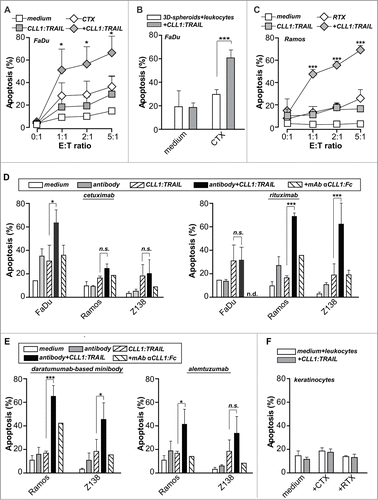
Figure 5 (See previous page). CLL1:TRAIL-armed leukocytes enhance the anti-cancer activity of therapeutic antibodies. (A) FaDu cells (EGFR positive) were incubated with different ratios of leukocytes and treated with cetuximab (CTX, 1 μg/ml), CLL1:TRAIL (200 ng/ml) combinations thereof. (B) FaDu cells cultured in 3D-spheroids were incubated with leukocytes (E:T = 10:1) and treated with cetuximab (CTX, 1 μg/ml), CLL1:TRAIL (200 ng/ml) or the combination. (C) Ramos cells (CD20 positive) were incubated with different ratios of leukocytes and treated with rituximab (RTX, 1 μg/ml), CLL1:TRAIL (200 ng/ml) or the combination. (D) As in A and C, using FaDu, Ramos and Z138 cells. In addition, αCLL1:Fc was used to block the effects of CLL1:TRAIL. (E) As in D, using Ramos and Z138 cells treated with the anti-CD38 antibody (daratumumab analog) and anti-CD52 antibody alemtuzumab (E:T ratio=5:1). (F) Primary normal human keratinocytes were treated with CLL1:TRAIL (700 ng/ml) and CTX or RTX (E:T ratio = 10:1). In all cases, apoptosis induction was determined using flow cytometric analysis of DioC6 staining. All graphs represent mean+SD. * p < 0.05, **p < 0.01, *** p < 0.001, n.s.: not significant, n.d.: not determined.