Figures & data
Figure 1. Schematic overview about the 3 major CrossMAb formats: CrossMAbFab, CrossMAbVH-VL and CrossMAbCH1-CL and predicted side products that can be formed. Mass spectrometry following transient expression and purification via protein A confirmed the presence of the predicted antibody species. In the case of CrossMAbFab, a non-functional monovalent heavy chain dimer is formed; in the case CrossMAbVH-VL, an antibody with a VL-CL Bence-Jones-like associated chain can occur. KiH technology or alternative heavy chain heterodimerization technologies applied where appropriate and indicated by colors only. Constant heavy chain domains are colored in gray, constant light chain domains in white, variable heavy chains are colored uniformly, light chain domains are colored with a line pattern.
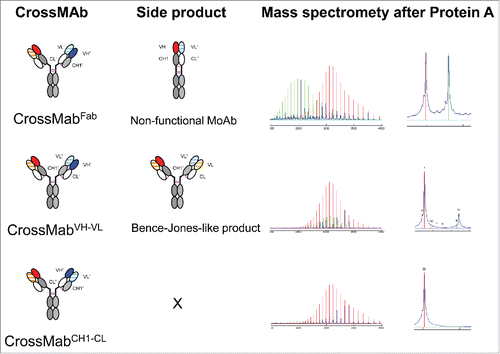
Figure 2. Overview of the clinical stage CrossMAbs: (A) Ang-2-VEGF CrossMAb vanucizumab (RG7112) for oncology, (B) VEGF-Ang-2 CrossMAb RG7716 for ophthalmology, (C) CEA TCB (RG7802) for CEA-positive solid tumors, (D) FAP-DR5 tetravalent CrossMAb RG7386. The black star depicts the P329G LALA (P329G, L235A, L234A) mutations to abolish FcγR and C1q binding, the green star Triple A (I253A, H310A, H435A) mutations to abolish FcRn binding. Constant heavy chain domains are colored in gray, constant light chain domains in white, variable heavy chains are colored uniformly, light chain domains are colored with a line pattern.
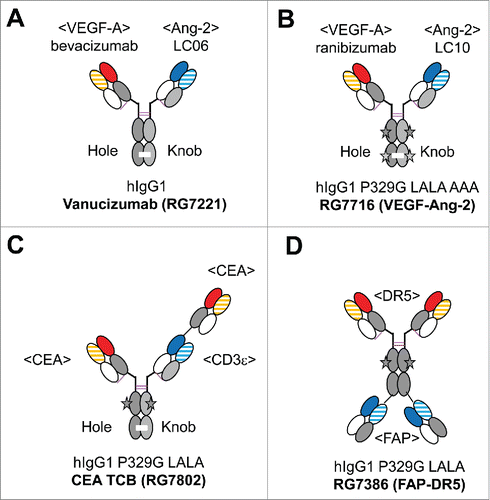
Figure 3. The CrossMAb zoo: Schematic overview about different mono-, bi- and multispecific antibody formats enabled by CrossMAb technology: (A) Heterodimeric/asymmetric trivalent 2+1 IgG CrossMAbs; (B) Symmetric tetravalent 2+2 IgG CrossMAbs; (C) MoAb (MonoMAb) and MoAb-Dimer (DuoMAb; (D) Trispecific Pan-HER family DAF-CrossMAb antibody; (E) Trispecific CrossMAb-VH-VL; (F) Tri-, tetraspecific CrossMAb-scFAb fusions; (G) DVD-CrossMAb; (H) Heterodimeric/asymmetric Kappa-Lambda-CrossMAb; (I) Fc-free Tandem Fab-CrossFab antibody. (J) Fc-free bispecific Fab fusion proteins using alternative fusion partners (green, yellow) based on Fab crossover. KiH technology or alternative heavy chain heterodimerization technologies applied where appropriate and indicated by colors only. The drawings given represent only examples, since in many cases crossed and uncrossed Fabs can be assembled in various ways. Constant heavy chain domains are colored in gray, constant light chain domains in white, variable heavy chains are colored uniformly, light chain domains are colored with a line pattern.
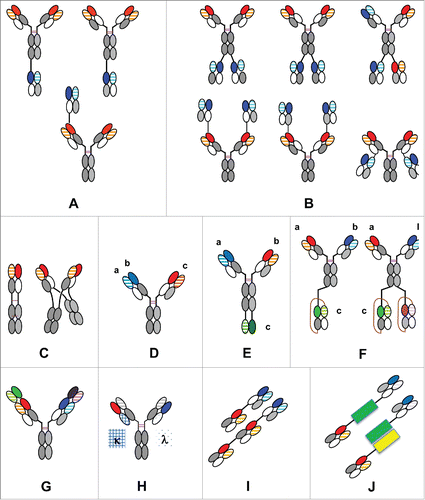
Figure 4. Design of CrossMAbs with CH1-CL crossover: Top: Typical structures of VH, Vk and Vλ domains and their superposition in the sense that the Cα atoms of the β-sheets adjacent to the elbow region (displayed as spheres in the structures, gray in the sequences) match in space. Middle: the same applied to the CH1, Cκ and Cλ domains. Bottom: wildtype and designed CrossMAb sequences; β-sheets adjacent to the elbow regions are colored gray. The newly designed sequence is shown in red.
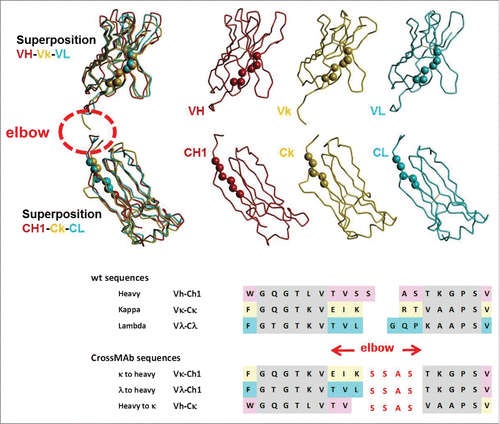
Table 1. Typical elbow sequences, * typically Cκ is used.
