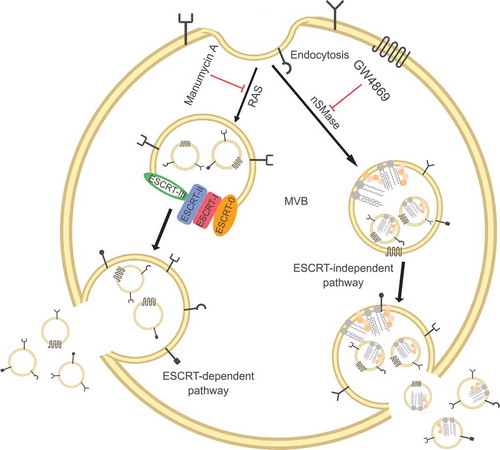
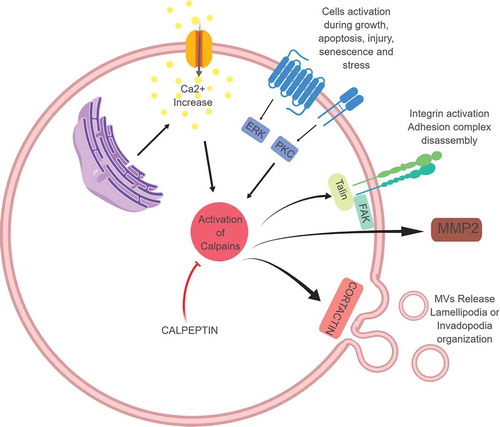
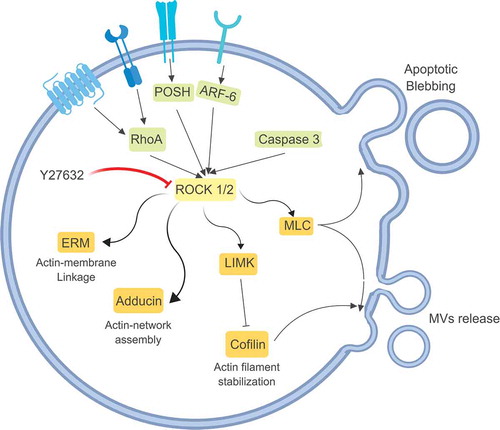
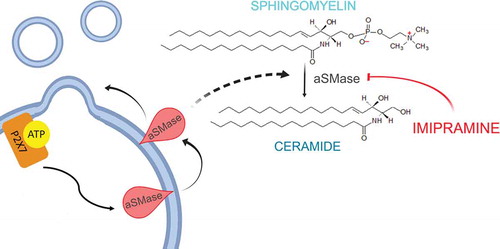
Figures & data
Table 1. Most commonly used compounds reported to prevent EVs (MVs or exosomes) release and their biological properties.
Table 2. EV separation and characterisation methods used in the studies discussed in this review.