Figures & data
Figure 1. Cell death kinetics and forms of B16 melanoma cells after irradiation and/or hyperthermia. The cell death forms of B16 mouse melanoma cells were analyzed with two color flow cytometry after staining with AnnexinV-FITC and DAPI 24, 48 or 72 h after the respective treatment with ionizing radiation with 15 Gy and/or hyperthermia (HT, 41.5°C for 1 h). Viable cells are defined as AnxV−/DAPI−, apoptotic cells as AnxV+/DAPI−and necrotic ones as AnxV+/DAPI+. Representative data of four independent experiments, each performed in triplicates, are presented as mean ± SD.
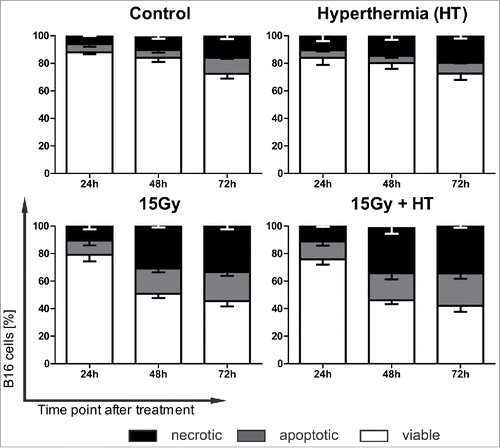
Figure 2. In vivo immunogenicity of treated B16 melanoma cells. B16 mouse melanoma cells were either irradiated with 15 Gy or additionally exposed to hyperthermia (HT, 41.5°C for 1 h) and injected 30 h later s.c. into the flank of syngenic C57BL/6 mice. After 7 d, viable B16 cells were injected in the contralateral flank (A). Tumor growth was followed after immunization with B16 cells pre-treated with RT (B), RT in combination with HT (C) and RT plus HT repetitively (rep.) twice the week (D) until day 25 at the side of injected viable B16 cells. Representative data of two independent experiments, each with five mice per group, are presented as mean ± SD ***p < 0.001 determined by two-way ANOVA, Bonferroni post-test; control: PBS immunized mice.
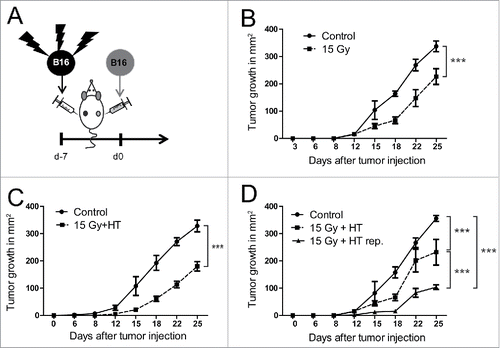
Figure 3. Immune cells in tumor draining lymph nodes of mice immunized repetitively with 15 Gy plus HT-treated B16 cells. Total cell count in tumor draining lymph nodes (sentinel) of C57BL/6 mice after repetitive (rep.) immunization with 15 Gy plus hyperthermia (HT, 41.5°C for 1 h) treated B16 cells is displayed in (A). The amount of infiltrated B cells (CD3−CD19+), NK cells (CD3−NK1.1+), T cell(CD3+)-subpopulations (NK1.1+, CD8+CD4−, CD8−CD4+, CD8+CD4+) is shown in (B) and that of NK cell-subpopulations (CD27+CD11b−, CD27+CD11b+, CD27−CD11b+) in (C). The analyses were performed by flow cytometry and are presented as mean ± SD **p < 0.01; ***p < 0.001 calculated by unpaired student's t-test.
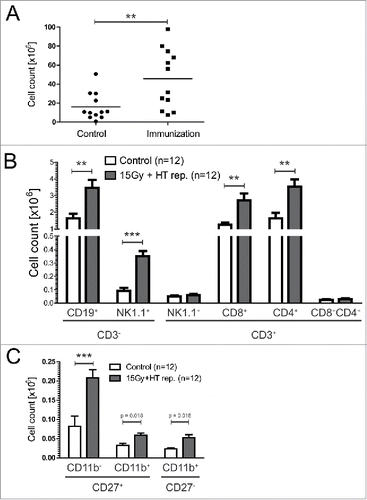
Figure 4. Impact of NK cells on tumor growth retardation induced by immunization with treated B16 cells. By repetitive immunization of C56/BL6 mice with 15 Gy plus hyperthermia (HT, 41.5°C for 1 h) treated B16 cells and subsequent injection of viable tumor cells in the contralateral flank, NK cells were either depleted before the first immunization (single NK-depl. before immunization), additionally three times after immunization (weekly NK-depl. after immunization), or before and additionally three times after immunization (weekly depletion) (A). Concomitantly the tumor growth was monitored over days at the side of injected viable B16 cells (B–C). ***p < 0.001 determined by two-way ANOVA, Bonferroni post-test. Additionally, the days to tumor growth >150 mm2 was recorded (D). *p < 0.05; **p < 0.01; ***p < 0.001 determined by one-way ANOVA, Bonferroni test. Representative data of two independent experiments, each with five mice per group, are presented as mean ± SD; control: PBS immunized mice.
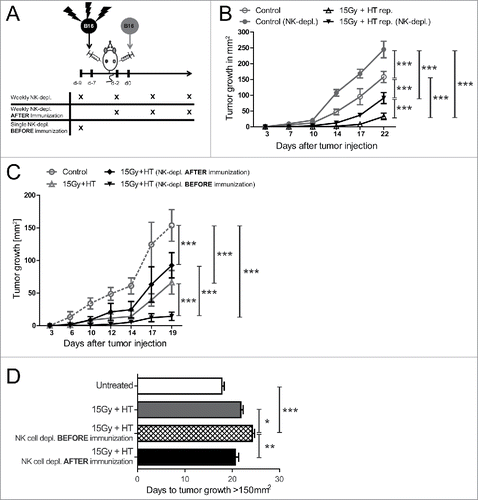
Figure 5. Impact of NK cells on immune cells in tumor draining lymph nodes of mice immunized with 15 Gy plus HT-treated B16 cells. Total cell count of immune cells (B cells (CD3−CD19+), NK cells (CD3−NK1.1+) and T cell(CD3+)-subpopulations (NK1.1+, CD8+CD4−, CD8−CD4+, CD8+CD4+)) in tumor-draining lymph nodes (sentinel) was determined in dependence of NK-depletion once before immunization. The analyses were performed by flow cytometry and are presented as mean ± SD **p < 0.01; ***p < 0.001 calculated by unpaired student's t-test.
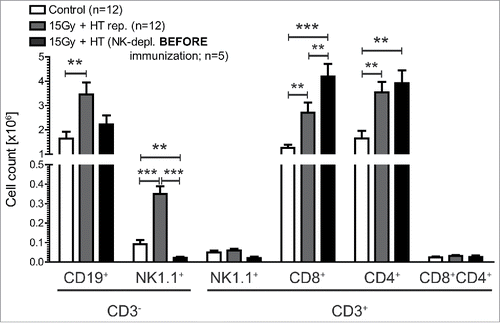
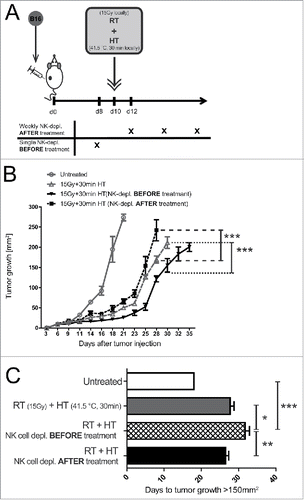
Figure 6. Impact of local treatment of established tumors with 15 Gy plus hyperthermia and NK cells on tumor growth retardation. Viable B16 tumor cells were injected into the flank of C57BL/6 mice. 10 d later, after tumor establishment, the tumors were locally irradiated with 15 Gy and 4 h later additionally locally treated with hyperthermia (HT, 41.5°C for 30 min). NK cells were either depleted weekly after the local treatments or 2 d before (A). Concomitantly, the tumor growth at was monitored over days (B–C). ***p < 0.001 determined by two-way ANOVA, Bonferroni post-test. Additionally, the days to tumor growth >150 mm2 was recorded (D). *p < 0.05; **p < 0.01; ***p < 0.001 determined by one-way ANOVA, Bonferroni test. Representative data of two independent experiments, each with five mice per group, are presented as mean ± SD.