Figures & data
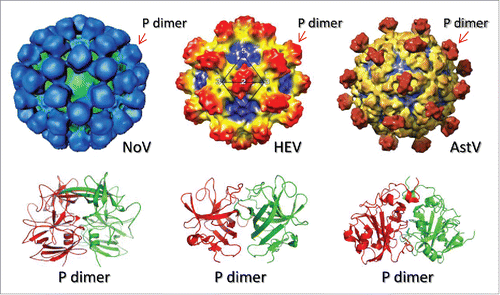
Figure 1. Schematic illustrations of a subviral particle formation (A) and its application as a polyvalent vaccine platform for combination subunit vaccine development (B). (A) Stepwise illustration of a subviral particle formation via homotypic interaction of the viral protein. The viral proteins are generated as monomers that can self-assemble into dimers and then a subviral particles via homotypic interaction of the viral proteins. Intermolecular disulfide bonds (red bars) may be introduced to stabilize the particle formation. (B) Application of the subviral particle as a polyvalent platform for a combination subunit vaccine development. A foreign antigen (red ball) is inserted to the top surface of the viral protein. Through dimerization and particle formation, multiple copies of the antigen are presented on the outermost surface of the subviral particle as a combination bivalent vaccine.
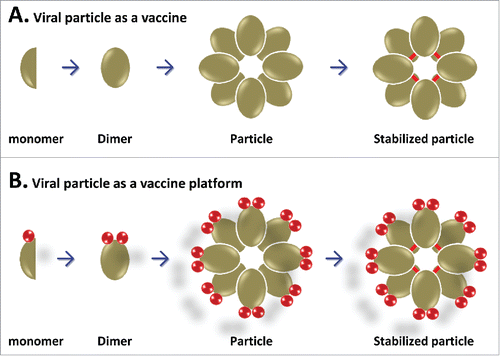
Figure 2. Schematic illustration of the formations of 3 viral protein polymers. (A) Lineage polymer formation. Fusion of 2 dimeric viral proteins (in green and blue, respectively) forms a lineage polymer via intermolecular dimerization of the homologous proteins. This lineage polymer can be used as a bivalent vaccine. (B) Network polymer formation. Fusions of 3 dimeric viral proteins (in green, blue, and purple, respectively) assembles into a network polymer via intermolecular dimerization of the homologous proteins. This network viral protein polymer can be used as a trivalent vaccine. (C) Agglomerate polymer formation. Fusion of a dimeric and an oligomeric viral proteins together assembles into an agglomerate polymer via intermolecular dimerization and oligomerization of the homologous proteins. This agglomerate polymer can be used as a bivalent vaccine.
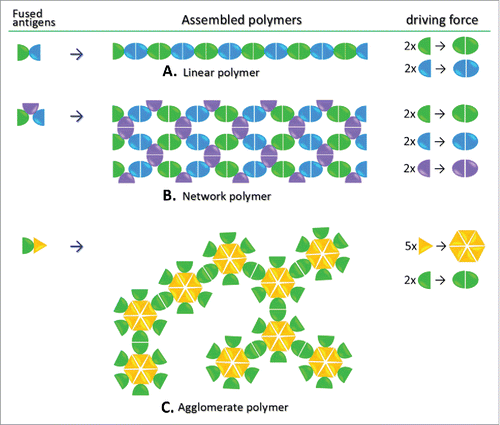
Figure 3. Schematic illustration of the common availability of viral dimeric surface proteins. The structures of norovirus (NoV), hepatitis E virus (HEV), and astrovirus (AstV) with indication of protruding (P) dimers on the capsid are shown in the top panel. The crystal structures of the P dimers are shown in the bottom panel. These dimeric viral proteins are ideal components for the viral protein polymer production.