Figures & data
Figure 1. The proportion of vaccine-type (VT) carriage of all carriage acquisition over time since vaccine introduction. Functions and
show the proportions of vaccine-type acquisition in the unvaccinated and vaccinated children, respectively. The scenario shown here corresponds to the observation in Finland. The initial proportion of VT carriage of all carriage among unvaccinated children at time
is
and decreases to
in about 70 months. The vaccine efficacy is
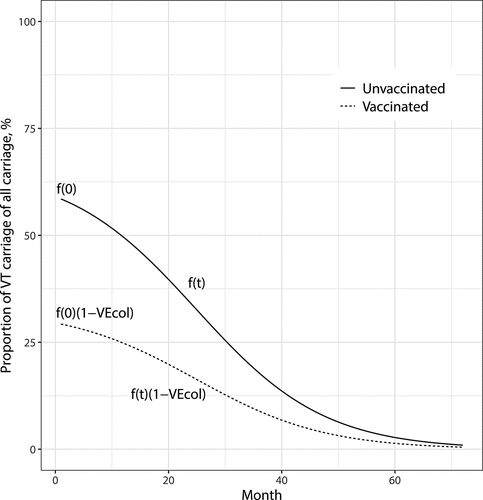
Table 1. Per capita rates of carriage acquisition at time after vaccine introduction per serotype category and vaccination status.
is the per capita rate of carriage acquisition,
is the proportion of VT carriage acquisition of all carriage, and
is the vaccine efficacy against carriage acquisition. Because of complete replacement in carriage, the rates of VT and NVT carriage acquisition sum up to
in all three columns of the table
Table 2. Per capita rates of IPD at time after vaccine introduction per serotype category and vaccination status.
is the rate of IPD before vaccine introduction and
is the vaccine efficacy against progression to disease
Table 3. The total and indirect impact of a vaccination program against VT, NVT, and all IPD in a before-after study setting
Table 4. Vaccine effectiveness against VT, NVT, and all IPD in a parallel study setting
Figure 2. Vaccine effectiveness and total and indirect impact as function of time since vaccination onset. Parameter values: ,
,
and the initial fraction of NVT IPD out of all IPD is
. VT carriage decreases to
in about 70 months. (a) Total impact (solid line) and effectiveness (dashed line) against VT; (b) total impact (solid line) and effectiveness (dashed line) against NVT; (c) total impact (solid line) and effectiveness (dashed line) against all IPD; (d) indirect impact against VT; (e) indirect impact against NVT; (f) indirect impact against all IPD
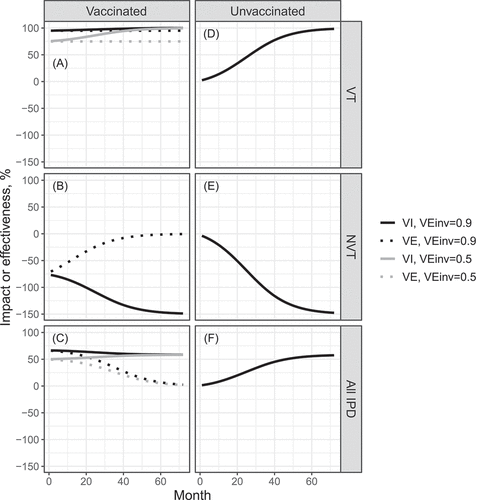
Figure 3. Overall impact against VT, NVT and all IPD as function of time since vaccination onset. Parameter values: proportion vaccinated ,
,
,
and the initial fraction of NVT IPD out of all IPD is
. VT carriage decreases to 0% in about 70 months
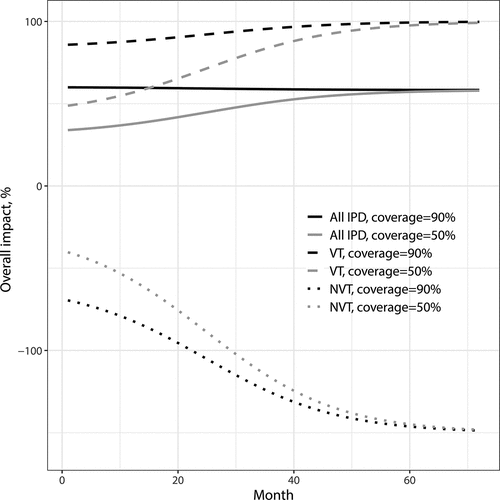
Figure 4. IPD cases and their vaccination status in the target cohort of PCV10 eligible children (years 2010–2016) and in the reference cohort (years 2004–2008) in Finland
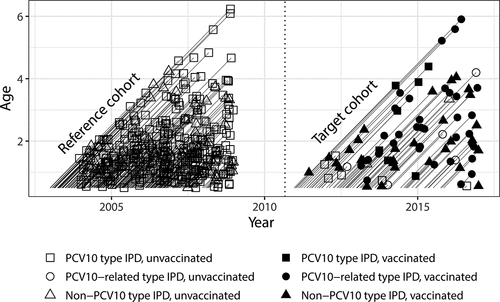
Figure 5. Overall impact against PCV10, PCV10-related and serotype 19A IPD among vaccine-eligible children in Finland in years 2012–2016
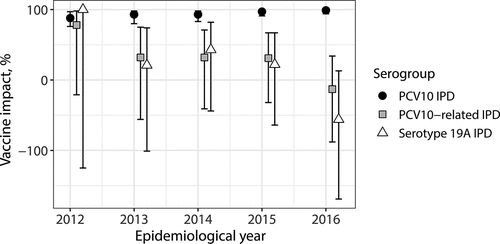
Table 5. Effectiveness of PCV10 vaccination in the PCV10-eligible target cohort (2010–2016), and the impact of PCV10 vaccination based on the comparison of the target cohort with a reference cohort (2002–2008), Finland
