Figures & data
Figure 1. Identification of membrane-trafficking factors involved in JEV infection. HBMEC were transfected with Human Membrane Trafficking siRNA library and infected with JEV (MOI of 10). Immunofluorescence assays were performed to detect JEV. The percentage of viral antigen-positive cells were calculated and normalized to siNT (non-targeting siRNA). Genes with 50% decrease in JEV infectivity (dotted line) compared to the siNT control were considered for further analysis. Transfection control: Tr-control; vehicle control: control.
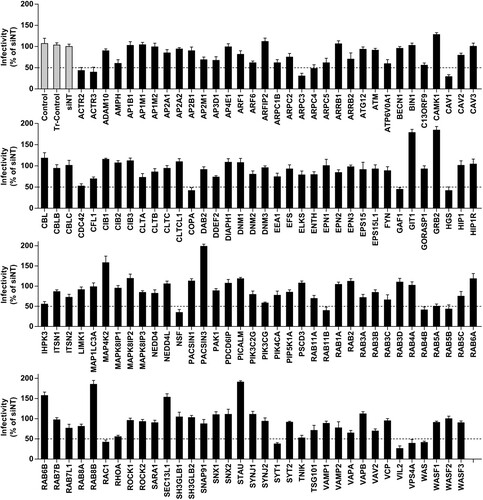
Figure 2. JEV entry into HBMEC via a caveolae-mediated endocytic pathway. Cells transfected with (A) siCLTC or (B) siCAV1 were incubated with JEV (MOI of 50), and internalization assays were performed. (C) Protein levels of clathrin heavy chain (CLTC) and caveolin-1 were measured by western blotting with GAPDH as reference. Values are normalized against that of non-targeting siRNA (siNT) control (A, B, C). (D) HBMEC were pre-treated with different concentrations of filipin III and infected with JEV (MOI of 10). JEV infectivity was measured by immunofluorescence. Cell viability was tested by the CCK-8 kit. (E) HBMEC were with and without (control) filipin III (20 μM) treatment were incubated with JEV (MOI of 50), and internalization assay was performed. Values were normalized against that of DMSO control (D, E). (F, G) Cells expressing wild-type (WT) or dominant-negative (DN) forms of caveolin-1 were infected with JEV (MOI of 10 or 50). Infectivity was analysed by immunofluorescence; intracellular JEV RNA, by internalization assay. Values are normalized against that of WT control. (H) AF-555 CT-B was added to cells pre-treated with filipin III, or transfected with siCAV1, or expressing caveolin-1 DN. The cells were examined by confocal microscopy. Scale bar, 200 μm. (I) Confocal microscopy localization of clathrin or caveolin-1 (red) with JEV (MOI of 100) (green). Arrowheads: JEV and caveolin-1 colocalization. Nuclei were DAPI-stained. Insets show magnified boxed areas. Scale bars, 20 μm. Results are representative of three independent experiments. **p < 0.01 compared to control.
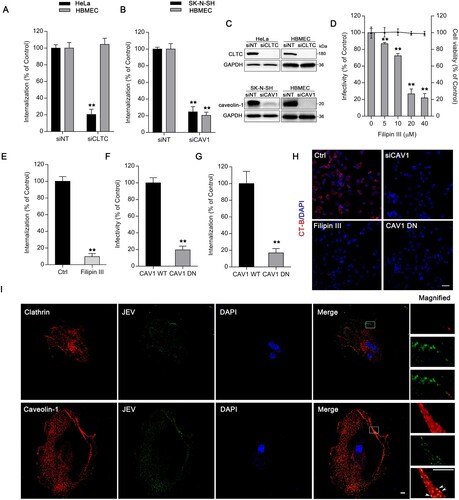
Figure 3. Ezrin-mediated actin reorganization is essential for JEV entry. HBMEC were transfected with siRNAs targeting ERM protein family members (EZR, MSN, RDX). (A) JEV (MOI of 10) infectivity and (B) intracellular JEV RNA (initial MOI of 50) were determined. Values were normalized against that of non-targeting siRNA (siNT) control. (C) HBMEC were treated with ezrin inhibitor NSC668394 at different concentrations and infected with JEV (MOI of 10) for 2 h. JEV infectivity was measured by immunofluorescence. Cell viability was tested by CCK-8 kit. (D) HBMEC were treated with and without (control) NSC668394 (30 μM) and incubated with JEV (MOI of 50); internalization assay was performed to analyse intracellular JEV RNA. (E) HBMEC bound with JEV (MOI of 10; 4°C, 2 h) were transferred to 37°C. At the indicated times (h.p.i, hours post-infection), cells were treated with NSC668394 (30 μM). Forty-eight hours after transfer to 37°C, JEV infectivity was analysed by immunofluorescence (−2: simultaneous addition of NSC668394 and JEV). (F,G) Cells were pre-treated with latrunculin A (Lat A, 5 μM), cytochalasin D (Cyt D, 10 μM), or jasplakinolide (Jas K, 5 μM), then infected with JEV (MOI of 10 or 50). JEV infectivity (F) and JEV internalization (G) were determined. Values were normalized against that of DMSO control (Ctrl; C-G). (G) Western blotting analysis shows fractions of G-actin and F-actin in JEV-infected HBMEC infected (MOI of 50) with or without NSC668394 (30 μM). The G-actin/F-actin ratio is shown below. Cells treated with DMSO were used as solvent control. (H) Cells exposed to JEV (MOI of 100) with or without NSC668394 (30 μM) for 2 h were stained with tetramethylrhodamine-conjugated phalloidin (red) and anti-JEV-NS3 (green) and observed by confocal microscopy. Arrowheads: colocalization of JEV and actin at membrane dorsal ruffles; arrows: non-overlapping JEV and actin on membrane. Nuclei were DAPI-stained. Insets show magnified boxed areas. Scale bars, 20 μm. Results are representative of three independent experiments. **p < 0.01 compared to control or indicated group (lined).
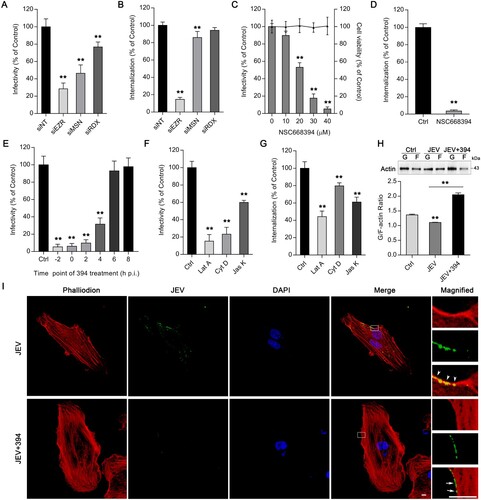
Figure 4. Ezrin is critical for Src/caveolin-1 interaction during JEV entry. HBMEC (A) transfected with siRNA targeting EGFR, RhoA, or Src, or (B) treated with PP2 at different concentrations were infected with JEV (MOI of 10). JEV infectivity was assessed by immunofluorescence. Cell viability was tested by CCK-8 kit. Dotted line: 50% JEV infectivity compared to the siNT control. HBMEC were (C) transfected with siSrc or (D) treated with PP2 (20 μM) and incubated with JEV (MOI of 50) and JEV internalization was determined. Values are normalized against that of non-targeting siRNA (siNT) or DMSO control (Ctrl) (A–D). (E–G) Western blot analyses of HBMEC cell lysates. (E) HBMEC were infected with JEV (MOI of 50) for 0.5, 1, 2, 4, 6 and blotted using an anti-p-ezrin, anti-ezrin, anti-p-Src, anti-Src, anti-p-caveolin-1 (p-cav-1), anti-caveolin-1 (cav-1), and anti-GAPDH (internal reference) antibodies. Ctrl: PBS control. (F) HBMEC were treated with NSC668394 (30 μM) or PP2 (20?M) and infected with JEV (MOI of 50) for 2 h. (G) HBMEC transfected with non-targeting siRNA (siNT) or siCAV1 were infected with JEV (MOI of 50) for 2 h. (H) Lysates from JEV-infected (MOI of 50) or PBS-treated HBMEC were subjected to immunoprecipitation (IP) with antibodies against Src, ezrin (ezr) or caveolin-1 (cav-1), or non-specific IgG controls. Immunoprecipitates were analysed by western blotting (WB) for the presence of phosphorylated proteins. (I, J) In vitro kinase assays: recombinant (I) ezrin, or (J) caveolin-1 was untreated, phosphorylated with recombinant Src or phosphorylated with Src, and further dephosphorylated with PP2 (1 μM). Half-reactions were analysed by western blotting using anti-p-ezrin or anti-p-caveolin-1 antibody; the other half were analysed using anti-ezrin or anti-caveolin-1 antibody. (K) Cells treated with DMSO control or NSC668394 (30 μM) were exposed to JEV (MOI of 100) for 2 h, stained with p-Src (red) and p-caveolin-1 (p-cav-1, green), and observed by confocal microscopy. Arrowheads: Src and caveolin-1 colocalization at the membrane ruffle. Nuclei: DAPI-stained. Insets show magnified boxed areas. Scale bars, 20 μm. Results are representative of three independent experiments. **p < 0.01 compared to control.
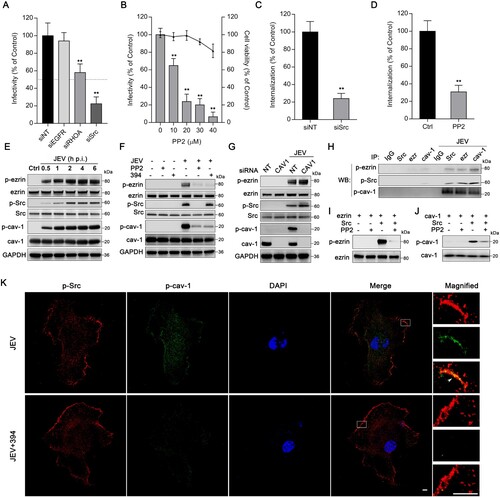
Figure 5. Inhibition of ezrin or Src protects mice from JEV infection-induced lethality. Mice were infected with JEV (4×103 PFU/mouse) and treated with PBS, NSC668394 (6 mg/kg) or PP2 (7.5 mg/kg) through the subcutaneous route. The drugs (include PBS) were continued daily for 5 days. (A) Kaplan-Meier survival curves for 14 days post-infection are shown. (B) JEV RNA in brain was quantified by qRT-PCR assay 5 days post-infection. Results are denoted as the relative quantity (RQ) of JEV RNA genome compared to the JEV (PBS) group. JEV, n = 7; JEV+394, n = 10; JEV + PP2, n = 9. (C) Mouse brain sections were stained for JEV-NS3 protein (green); nuclei were stained with DAPI. Insets show magnified boxed areas. Scale bars in scanned and magnified images are 500 and 50 μm, respectively. Results are representative of three independent experiments. *p < 0.05 compared to the JEV-infected, PBS-treated group. **p < 0.01 compared to the JEV-infected, PBS-treated group.
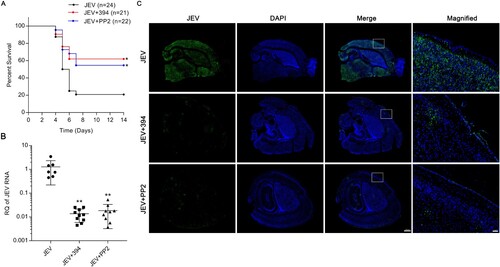
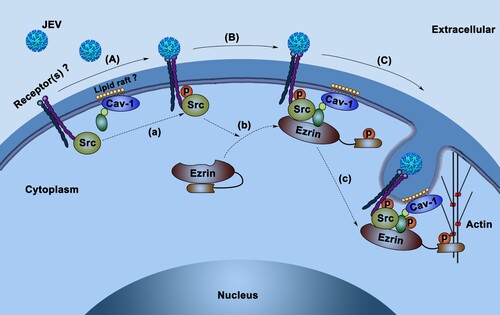
Figure 6. Proposed model of JEV entry route upon HBMEC infection. (A) JEV attaches to HBMEC surface receptors (e.g. integrins), co-receptors, or other attachment proteins, leading to the phosphorylation of Src (a). (B) Phosphorylated-Src induces phosphorylation and activation of ezrin (b). Ezrin in its open conformation (p-ezrin) anchors p-Src to the caveolin-1-enriched region (probably a lipid raft). (C) p-Src induces the phosphorylation and activation of caveolin-1 on the scaffold of p-ezrin (c) and mediates the formation of endocytic carriers in synergy with ezrin-mediated actin polymerization.