Figures & data
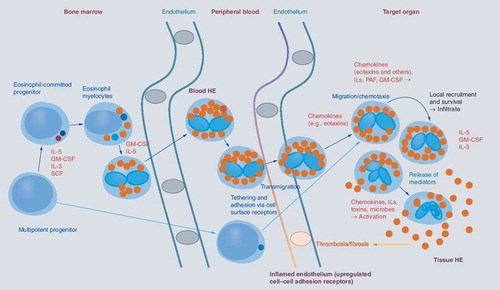
Eosinophils originate from multipotent and lineage-restricted hematopoietic progenitor cells. Eosinophil progenitors reside in the bone marrow but are also detectable in the peripheral blood. Eosinophil development is regulated by eosinophilopoietic cytokines (IL-3, GM-CSF and IL-5) and takes place primarily in the bone marrow. Cytokine-induced HE in the peripheral blood is often accompanied by tissue HE. Activation of eosinophils and activation of endothelial cells contribute to endothelial transmigration and infiltration of inflamed tissues. Eosinophil adhesion to endothelium and transmigration are mediated by certain homing receptors. Migration and accumulation of eosinophils in (inflamed) tissues are mediated by chemotactic peptides (chemokines), cytokines and other mediators. Eosinophil accumulation is also triggered by delayed eosinophil apoptosis, another cytokine-mediated phenomenon, in local tissue sites. Activation of eosinophils leads to degranulation and mediator secretion in tissues, with consequent organ damage, which may be accompanied by fibrosis and/or thrombosis and by deposition of eosinophil granule proteins. HE: Hypereosinophilia; GM-CSF: Granulocyte/macrophage colony-stimulating factor; IL: Interleukin; PAF: Platelet-activating factor; SCF: Stem cell factor.
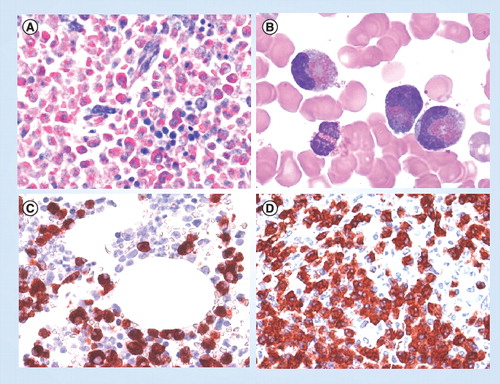
(A) Giemsa-stained bone marrow section in a patient with eosinophilic leukemia. Note the massive eosinophil infiltrate replacing the normal bone marrow. (B) Circulating immature eosinophils in a patient with FIP1L1/PDGFRA+ chronic eosinophilic leukemia. (C) & (D) Bone marrow biopsy sections obtained from a patient with FIP1L1/PDGFRA+ chronic eosinophilic leukemia. Sections were incubated with an antibody directed against eosinophil major basic protein and analyzed by indirect immunohistochemistry.
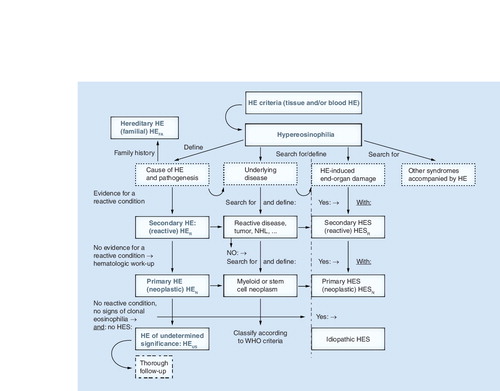
After having established that HE is present, the cause and clinical significance of HE need to be explored. With regard to the cause, the patient is examined for signs of a reactive process (helminth infection, drug allergy or others), evidence of a myeloid or stem cell neoplasm (where eosinophils usually are neoplastic cells), or of other malignancies. In rare cases, familial HE is diagnosed. When no underlying condition and no signs of overt organ damage are found, the (provisional) diagnosis HEUS is established, and the patient is carefully monitored. In the case of secondary (reactive or paraneoplastic) HE or clonal (neoplastic) HE, the final diagnosis is determined using the WHO criteria and other established criteria. When HE is accompanied by specific (eosinophil-induced) organ damage, diagnosis of HES is established. HES can occur in any type of HE and can present as secondary (reactive) HES, primary (neoplastic) HES, or idiopathic HES. Rare syndromes presenting with eosinophilia, such as the CCS, Gleich’s syndrome and other syndromes, must also be considered based on clinical findings. CCS: Churg–Strauss syndrome; HE: Hypereosinophilia; HEN: Clonal/neoplastic HE; HER: Reactive HE; HEUS: HE of undetermined significance; HES: Hypereosinophilic syndrome; HESN: Primary/neoplastic HES; HESR: Reactive HES; NHL: Non-Hodgkin´s lymphoma.