Figures & data
Figure 1 DLX6-AS1 is upregulated in PC tissues and cell lines.
Notes: (A) The mRNA expression of DLX6-AS1 in PC tissues and adjacent non-tumor tissues of 60 PC patients was determined by qRT-PCR. DLX6-AS1 was highly expressed in PC tissues. (B) Five-year survival rate of PC patients with high (n=28) and low (n=32) DLX6-AS1 expression was analyzed by Kaplan–Meier method and log-rank test. The patients with a low DLX6-AS1 expression level (less than or equal to median, n=32) presented better survival rates compared with those with a high DLX6-AS1 expression level (greater than or equal to median, n=28). (C) The mRNA expression level of DLX6-AS1 in normal HPDE cells and six PC cell lines (Panc-1, Bxpc-3, AsPC-1, Capan-1, CFPAC-1, and MIA PaCa-2) was determined by qRT-PCR. DLX6-AS1 expression was significantly higher in all the PC cell lines than HPDE cells. Data are presented as mean ± SD. ***P<0.001 vs HPDE cells.
Abbreviations: HPDE, human pancreatic ductal epithelial; PC, pancreatic cancer; qRT-PCR, quantitative reverse transcription PCR.
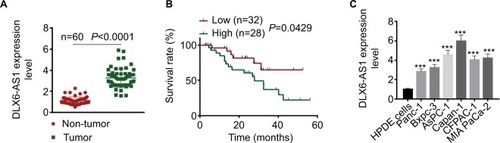
Figure 2 DLX6-AS1 promotes proliferation and cell cycle progression and inhibits apoptosis of PC cells.
Notes: (A) The expression efficiency of DLX6-AS1 vectors. pcDNA3.1-DLX6-AS1 increased the expression of DLX6-AS1, and pLKO.1-DLX6-AS1 decreased the expression of DLX6-AS1. (B) The proliferation of PC cells was detected by CCK-8 assay. Overexpression of DLX6-AS1 promoted proliferation of PC cells, while knockdown of DLX6-AS1 inhibited proliferation of PC cells. (C) The apoptosis and (D) cell cycle progression of PC cells were detected by flow cytometry. Overexpression of DLX6-AS1 reduced the apoptosis rate and promoted the cell cycle progression, whereas knockdown of DLX6-AS1 increased the apoptosis rate and inhibited the cell cycle progression. (E) The expression of apoptosis-related proteins, Bcl-2 and cleaved caspase 3, and cell cycle-related proteins, cyclin D1 and p27, in PC cells was detected by Western blot. Data are presented as mean ± SD. *P<0.05 and ***P<0.001.
Abbreviations: CCK-8, Cell Counting Kit-8; PC, pancreatic cancer.
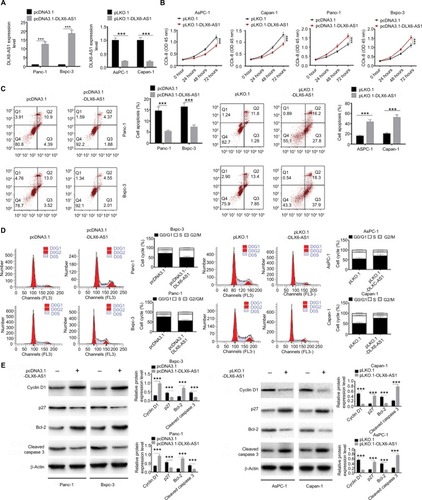
Figure 3 DLX6-AS1 promotes migration and invasion of PC cells.
Notes: (A) The migration of PC cells was detected by wound healing assay. Overexpression of DLX6-AS1 significantly accelerated the wound area closure, while the wound healing was inhibited by knockdown of DLX6-AS1. (B) The invasion of PC cells was detected by Transwell chamber assay. Overexpression of DLX6-AS1 promoted the invasive ability of PC cells, while the invasive ability was reduced by knockdown of DLX6-AS1. (C) The expression of EMT-associated proteins, E-cadherin, and MMP-2, in PC cells was detected by Western blot. Overexpression of DLX6-AS1 decreased E-cadherin level and increased MMP-2 level, while knockdown of DLX6-AS1 increased E-cadherin level and decreased MMP-2 level. Data are presented as mean ± SD. ***P<0.001.
Abbreviations: EMT, epithelial–mesenchymal transition; PC, pancreatic cancer.
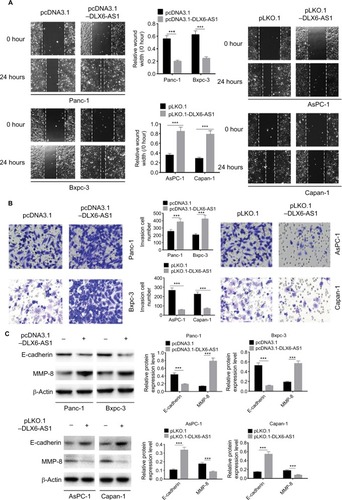
Figure 4 DLX6-AS1 promotes PC growth and metastasis in vivo.
Notes: (A) A subcutaneous PC tumor model was established. Tumor pictures are given on the left, volume data in the middle, and weight on the right. Overexpression of DLX6-AS1 promoted the growth of tumor, while knockdown of DLX6-AS1 inhibited the growth of tumor. (B) Above is the H&E picture of lung metastatic foci, and below is the H&E picture of liver metastatic foci. Knockdown of DLX6-AS1 inhibited the metastatic behavior of PC cells by reducing the number of metastatic tumors in the lung and liver, while overexpression of DLX6-AS1 increased the number of metastatic tumors in the lung and liver. Data are presented as mean ± SD. ***P<0.001.
Abbreviation: PC, pancreatic cancer.
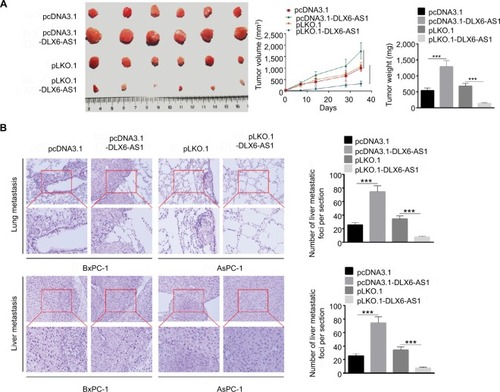
Figure 5 miR-497-5p targets DLX6-AS1 and negatively correlates with DLX6-AS1 expression.
Notes: (A) The possible target of DLX6-AS1 was predicted by starBase v2.0. The DLX6-AS1 binds the sites in the 3′-UTR of miR-497-5p. (B) The mRNA expression of miR-497-5p was detected by qRT-PCR. miR-497-5p was downregulated in PC tissues compared with adjacent normal tissues. (C) miR-497-5p was negatively correlated with DLX6-AS1 expression. (D) The binding of miR-497-5p and DLX6-AS1 was validated by RNA pull-down assay. DLX6-AS1 overexpression vector presented a higher miR-497-5p enrichment than that of the control. (E) The binding of miR-497-5p and DLX6-AS1 was validated by dual-luciferase reporter assay. Unlike negative control (NC) mimics, miR-497-5p mimics reduced the luciferase activity of wild-type (WT) DLX6-AS1 reporter vector but not that of mutant (MUT) one. (F) Overexpression of DLX6-AS1 decreased miR-497-5p level, whereas knockdown of DLX6-AS1 increased miR-497-5p level. Data are presented as mean ± SD. ***P<0.001.
Abbreviations: PC, pancreatic cancer; qRT-PCR, quantitative reverse transcription PCR.
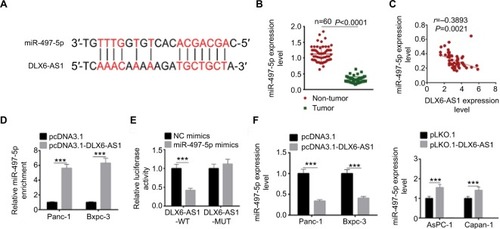
Figure 6 miR-497-5p targets FZD4 and FZD6 and inhibits Wnt/β-catenin signaling pathway by decreasing the expression of FZD4 and FZD6.
Notes: (A) The putative miR-497-5p-binding sites in the 3′-UTR of FZD4 and FZD6 were predicted by TargetScan. (B) The binding of miR-497-5p and FZD4 or FZD6 was validated by dual-luciferase reporter assay. Unlike negative control (NC) mimics, miR-497-5p mimics reduced the luciferase activity of wild-type (WT) FDZ4 and FDZ6 reporter vectors, but not that of mutant (MUT) reporter vectors. (C) Overexpression of miR-497-5p decreased the expression of FZD4, FZD6, and β-catenin, while knockdown of miR-497-5p increased the expression of FZD4, FZD6, and β-catenin. (D) The overexpression efficiency of pcDNA3.1-FZD4 and pcDNA3.1-FZD6 was determined by qRT-PCR and Western blot. (E) miR-497-5p mimics decreased the expression of FZD4, FZD6, and β-catenin, while overexpression vectors of FZD4 or FZD6 abrogated the regulation effect of miR-497-5p on FZD4 or FZD6. The expression of β-catenin was abrogated by either overexpression vectors of FZD4 or FZD6. Data are presented as mean ± SD. ***P<0.001.
Abbreviation: qRT-PCR, quantitative reverse transcription PCR.
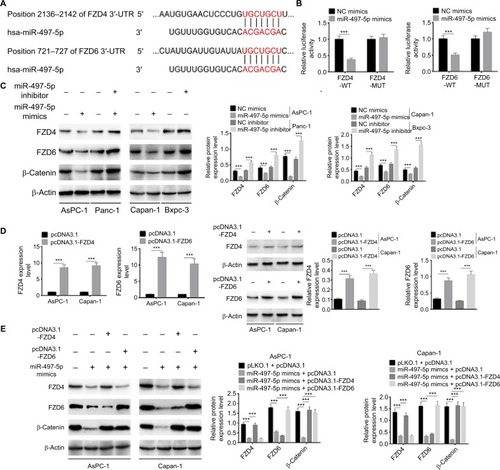
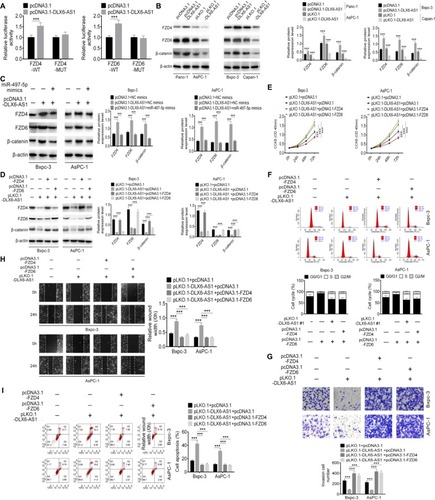
Figure 7 DLX6-AS1 promotes PC cell proliferation, migration, and invasion via miR-497-5p/FZD4/FZD6/Wnt/β-catenin signaling pathway.
Notes: (A) The effect of DLX6-AS1 on FZD4 and FZD6 was determined by dual-luciferase reporter assay. (B) Overexpression of DLX6-AS1 increased the expression of FZD4, FZD6, and β-catenin, whereas knockdown of DLX6-AS1 decreased the expression of FZD4, FZD6, and β-catenin. (C) Overexpression of DLX6-AS1 increased the expression of FZD4, FZD6, and β-catenin, while this increasing effect was abrogated by miR-497-5p mimics. (D) Knockdown of DLX6-AS1 inhibited the expression of FZD4, FZD6, and β-catenin, while this inhibitory effect was abrogated by the overexpression vectors of FZD4 or FZD6. Knockdown of DLX6-AS1 inhibited (E) proliferation, (F) cell cycle, (G) migration, and (H) invasion, and promoted (I) apoptosis of PC cells, while these inhibition or promotion effects of DLX6-AS1 were abrogated by the overexpression vectors of FZD4 or FZD6. Data are presented as mean ± SD. **P<0.01, and ***P<0.001.
Abbreviations: CCK-8, Cell Counting Kit-8; PC, pancreatic cancer.