Figures & data
Figure 1 Included patients.
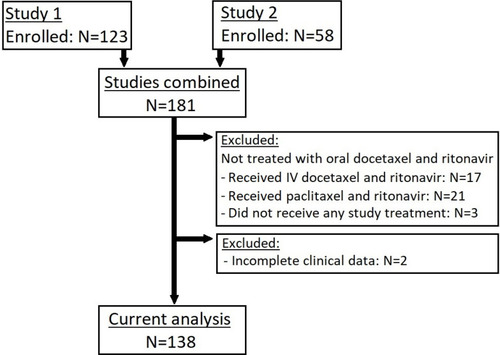
Table 1 Characteristics of the Patients Evaluable for Toxicity
Figure 2 Duration of treatment.
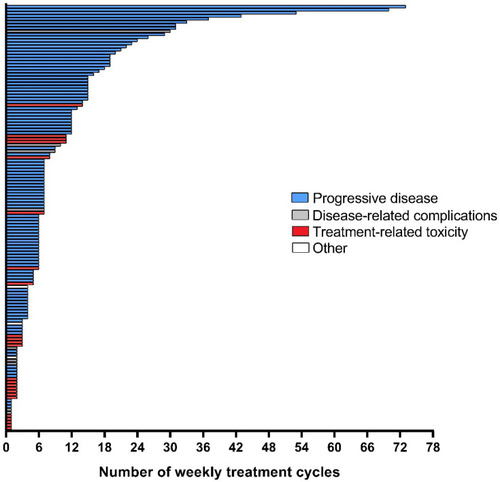
Table 2 Severe Toxicities Related to Oral Docetaxel Co-Administrated with Ritonavir
Figure 3 Correlation of the Cmax and the AUC of docetaxel.
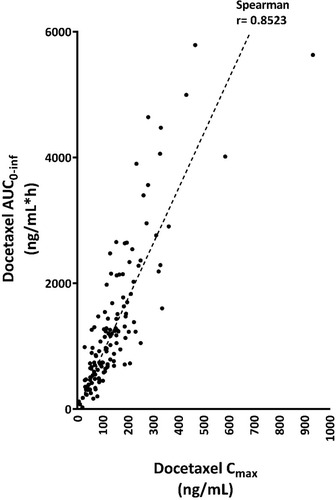
Figure 4 Relation of the exposure to ritonavir versus docetaxel.
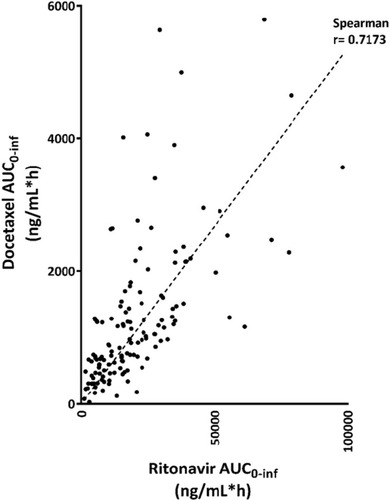
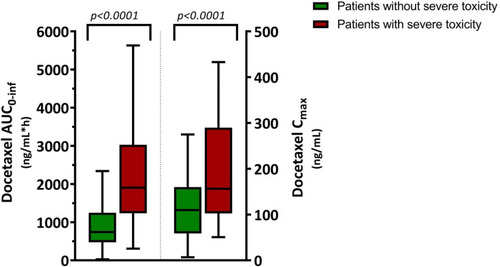
Figure 5 Pharmacokinetic exposure to docetaxel in patients with and without severe toxicity.