Figures & data
Figure 1 Protein synthesis.
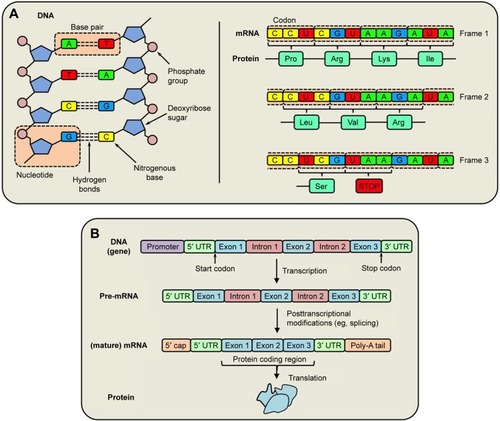
Figure 2 Mechanisms of oligonucleotides.
Abbreviations: ASO, antisense oligonucleotide; RISC, RNA-induced silencing complexes.
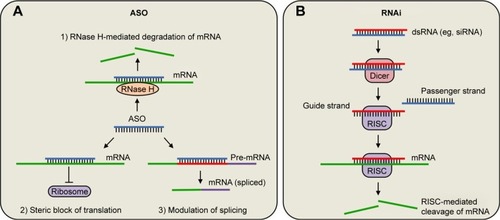
Figure 3 Pharmacodynamic effects of IONIS-TTRRX in healthy volunteers.
Abbreviation: chg, change.
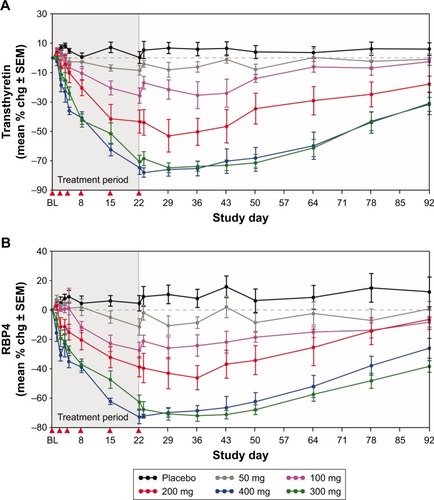
Figure 4 Primary end points: Inotersen Phase III study.
Abbreviations: mNIS +7, modified Neuropathy Impairment Score +7; QOL-DN, Quality of Life Questionnaire-Diabetic Neuropathy.
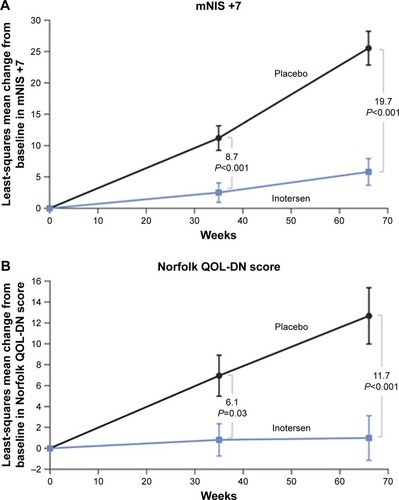
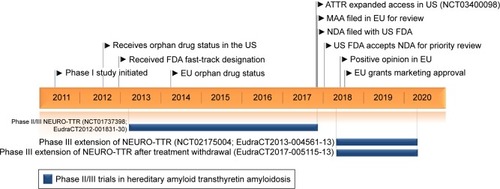
Figure 5 Inotersen timeline.
Abbreviations: ATTR, TTR amyloidosis; US FDA, US Food and Drug Administration; MAA, marketing authorization application; NDA, new drug application.