Figures & data
Figure 1 Study Design of the single ascending dose (SAD) study (A), and multiple ascending dose (MAD) study (B).
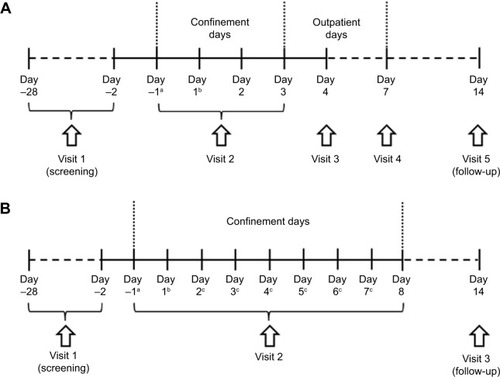
Table 1 Demographic characteristics of the study subjects
Table 2 Pharmacokinetic parameters of KM-819 in the SAD study
Figure 2 Mean plasma concentration-time profile of KM-819 in the single ascending dose (SAD) (A) and multiple ascending dose (MAD) (B) studies.
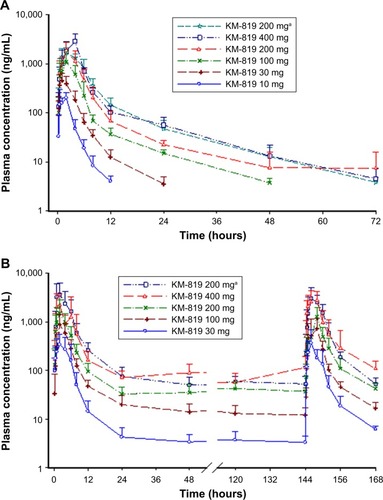
Table 3 Pharmacokinetic parameters of KM-819 in the MAD study
Table 4 Assessment of dose proportionality of KM-819 pharmacokinetic parameters (power model)
Table 5 Treatment emergent adverse events in the SAD and MAD studies
Table S1 Inclusion and exclusion criteria for KM-819 first-in-human study
Table S2 Summary of change from baseline (day 1 predose) to day 7 for PD parameters
Data availability
The raw data of this study will not be shared because of confidentiality.
