Figures & data
Table 1 Demographic Characteristics and Other Baseline Values of Test-Reference Treatment (T-R) and Reference-Test Treatment (R-T) Groups Under Fasting and Fed Conditions
Table 2 The Main Pharmacokinetic Parameters After Oral Administration of 20 Mg Escitalopram Oxalate Tablets Under Fasting and Fed Conditions
Table 3 Statistical Comparison After Ln-Transformed Cmax, AUC0-t and AUC0-∞of the Test and Reference Formulations Under Fasting Conditions (n = 21)
Table 4 Statistical Comparison After Ln-Transformed Cmax, AUC0-t and AUC0-∞ of the Test and Reference Formulations Under Fed Conditions (n = 19)
Table 5 ANOVA Test After Ln-Transformed Cmax, AUC0-t and AUC0-∞ of the Test and Reference Formulations Under Fasting and Fed Conditions
Figure 2 Mean plasma concentration-time profiles after oral administration of 20 mg escitalopram oxalate tablets of the test and reference formulations under fasting conditions.
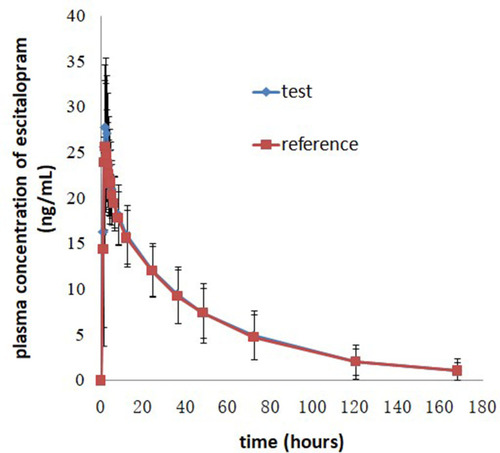
Figure 3 Mean plasma concentration-time profiles after oral administration of 20 mg escitalopram oxalate tablets of the test and reference formulations under fed conditions.
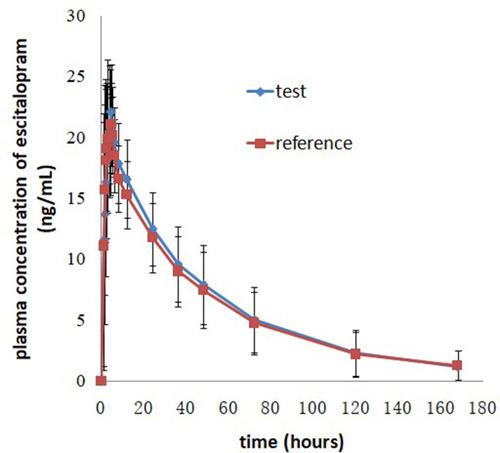
Table 6 The Incidence Rate and Comparison of AEs for the Two Formulations Under Fasting and Fed Conditions
Table 7 Summary of AEs for the Test and Reference Formulations Under Fasting and Fed Conditions