Figures & data
Table 1 In Vitro Accelerated Stability Test Results for Fixed-Dose Combination of Gemigliptin/Rosuvastatin 50/20 mg as Bilayer Tablet or Monolayer Tablet
Table 2 Pharmacokinetic Parameters of Gemigliptin, LC15-0636 and Rosuvastatin After a Single Administration of Fixed-Dose Combination of Gemigliptin/Rosuvastatin 50/20 mg as Bilayer Tablet or Monolayer Tablet
Figure 1 Mean plasma concentration–time profiles of (A) gemigliptin, (B) LC15-0636 and (C) rosuvastatin after a single administration of fixed-dose combination of gemigliptin/rosuvastatin 50/20 mg as bilayer tablet or monolayer tablet.
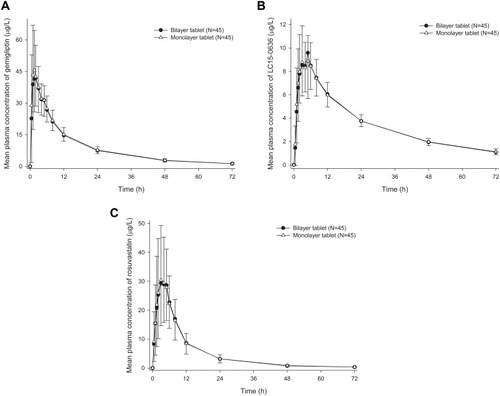
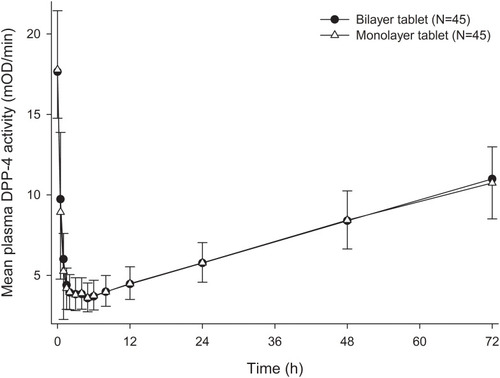
Table 3 Plasma Dipeptidyl Peptidase-4 (DPP-4) Activity Inhibition from Baseline After a Single Administration of Fixed-Dose Combination of Gemigliptin/Rosuvastatin 50/20 mg as Bilayer Tablet or Monolayer Tablet