Figures & data
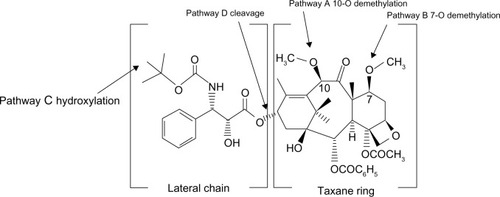
Figure 1 Chemical structure of 10-deacetylbaccatin III, docetaxel, and cabazitaxel.
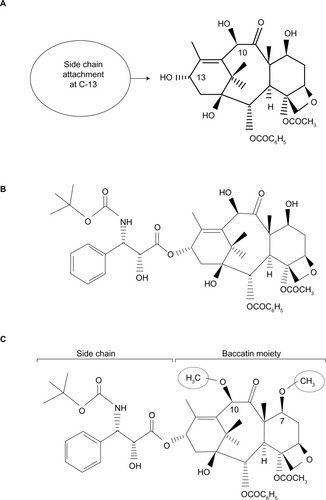
Table 1 In vitro antiproliferative effects of cabazitaxel and docetaxel against sensitive and P-glycoprotein-expressing resistant cell lines
Table 2 Pharmacokinetic parameters of cabazitaxel in normal and tumor-bearing mice, rats, and dogs
Figure 2 Pharmacokinetics of 40 mg/kg cabazitaxel (highest nontoxic dose) in plasma and tumor tissues in mice.
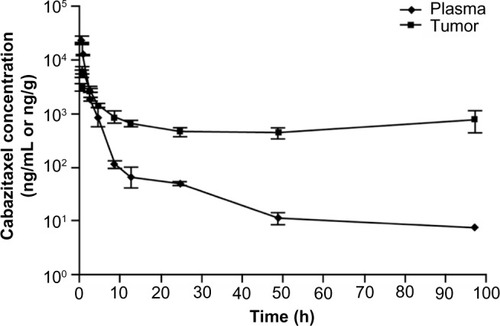
Table 3 Relative contribution of different metabolic pathways to cabazitaxel metabolism across species
Figure 4 Antitumor activity of cabazitaxel, docetaxel, and abiraterone in a docetaxel-sensitive hormone-refractory prostate cancer xenograft model.
Abbreviation: SEM, standard error of the mean.
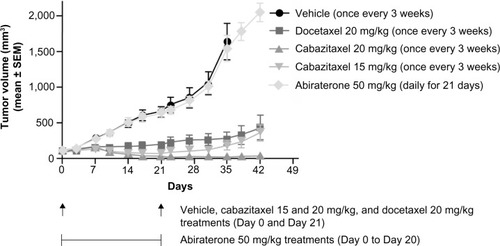
Table 4 Dose–response antitumor activity of cabazitaxel and docetaxel in mice bearing murine and human docetaxel-sensitive and -resistant tumors
Table 5 Cabazitaxel preclinical toxicology data summary: main toxicity parameters (mg/kg/day) in mice, rats, and dogs
Table 6 Cabazitaxel preclinical toxicology data summary: NOEL (mg/kg/day) for main target organs in rat and dog toxicity studies
Table 7 Cabazitaxel preclinical toxicology data summary: NOEL (mg/kg/day) for central and peripheral neurotoxicity in mice and rats
Figure 5 Mouse brain histopathology and autoradiography.
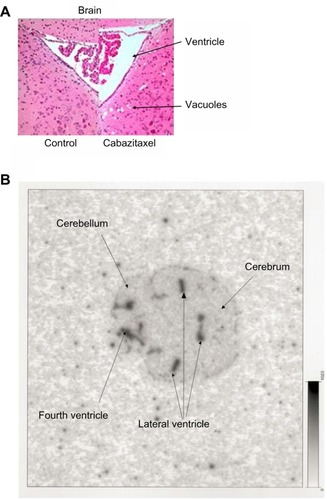
Table 8 Comparison of HNLDs (mg/kg) in single-dose toxicity studies in mice, rats, and dogs with cabazitaxel and docetaxel
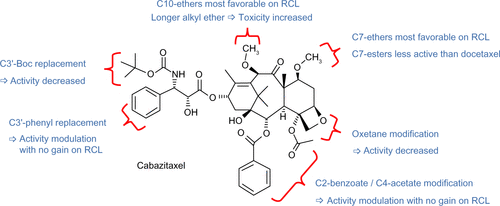
Figure S1 Structure–activity relationships for cabazitaxel.
Abbreviation: RCL, resistant cell line.