Figures & data
Table 1 Sequences of primers designed for real-time polymerase chain reaction analysis
Figure 1 The chromatograms of schisandrin (Sch) A and SchB, two major active components of Schisandra chinensis extract under high-performance liquid chromatography analysis. The column was eluted using H2O:methanol (v/v, 30:70) at a flow rate of 1.0 mL/minute for 35 minutes. The absorbance at 254 nm was monitored.
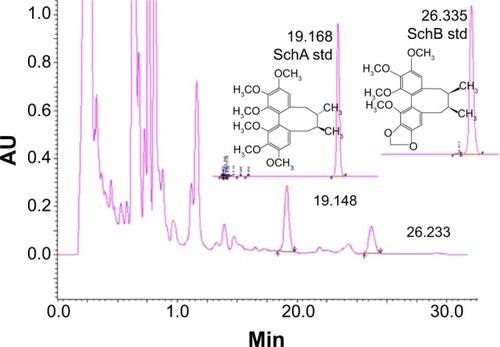
Figure 2 Effect of Schisandra chinensis extract, SchA, and SchB on the viability of human hepatocellular liver carcinoma cell line cells. Human hepatocellular liver carcinoma cell line cells were treated with S. chinensis extract at 15–480 µg/mL (A), SchA at 3.125–100 µM, and SchB at 12.5–400 µM for 24 hours. The effect of tBHQ, a known nuclear factor (erythroid-derived 2)-like 2 activator, at 3.125–100 µM on cell viability, was also examined (B). Cell viability was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The data shown are the mean ± standard deviation of at least three independent experiments.
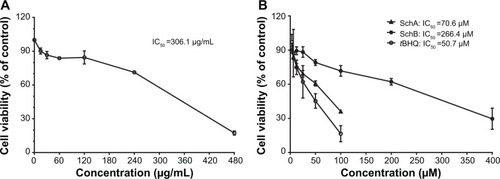
Figure 3 Effect of Schisandra chinensis extract (SCE), schisandrin (Sch) A, and SchB on the expression of NAD(P)H:quinone oxidoreductase 1 (NQO1), glutamate–cysteine ligase, modifier subunit (GCLM), heme oxygenase-1 (HO-1), and glutathione S-transferase A4 (GSTA4) in human hepatocellular liver carcinoma cell line cells. Human hepatocellular liver carcinoma cell line cells were treated with SCE at 100–300 µg/mL, 20 µM SchA, or 200 µM SchB for 24 hours. Total ribonucleic acid was extracted from the treated cells and then the first-strand complementary deoxyribonucleic acid was synthesized. A quota of complementary deoxyribonucleic acid (2 µL) was used as the template for quantitative polymerase chain reaction analysis of the target genes in each sample. (A–D) Bar graphs show the messenger ribonucleic acid expression levels of NQO1, GCLM, HO-1, and GSTA4, respectively. Data are presented as the mean ± standard deviation (n=3).
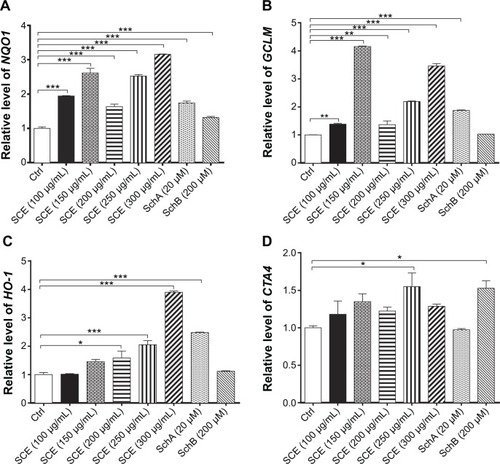
Figure 4 Effect of SCE, SchA, and SchB on the expression of HO-1 and GCLM in human hepatocellular liver carcinoma cell line cells. Human hepatocellular liver carcinoma cell line cells were treated with SCE at 100–300 µg/mL, 20 µM SchA, or 200 µM SchB for 24 hours. Cellular lysates were subject to Western blotting assay. (A) Representative blots of HO-1 and GCLM. (B, C) Bar graphs to show the expression levels of HO-1 and GCLM, respectively. Nicotinamide adenine dinucleotide phosphate was used as the internal control. Data are presented as the mean ± standard deviation from at least three independent experiments.
Abbreviations: SCE, Schisandra chinensis extract; Sch, schisandrin; HO-1, heme oxygenase-1; GCLM, glutamate–cysteine ligase, modifier subunit; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Ctrl, control.
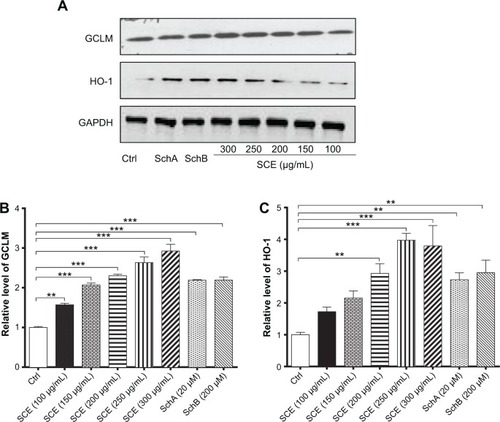
Figure 5 Effect of Schisandra chinensis extract (SCE), schisandrin (Sch) A, and SchB on the messenger ribonucleic acid expression of P-glycoprotein (P-gp), multidrug resistance-associated protein (MRP) 2, MRP4, organic anion transporting peptide (OATP) 1A2, and OATP1B1 in human hepatocellular liver carcinoma cell line cells. Human hepatocellular liver carcinoma cell line cells were treated with SCE at 100–300 µg/mL, 20 µM SchA, or 200 µM SchB for 24 hours. Total ribonucleic acid was extracted and complementary deoxyribonucleic acid was synthesized. A quota of complementary deoxyribonucleic acid (2 µL) was used for real-time polymerase chain reaction analysis of the target genes. (A–E) Bar graphs to show the relative expression levels of P-gp, MRP2, MRP4, OATP1A2, and OATP1B1, respectively. tert-Butylhydroquinone (tBHQ) was used as a known nuclear factor (erythroid-derived 2)-like 2 activator. Data are presented as the mean ± standard deviation (n=3).
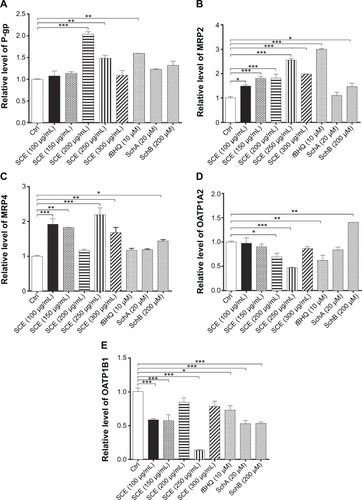
Figure 6 Effect of SCE, SchA, and SchB on the protein expression of P-gp, MRP2, and OATP1A2 in human hepatocellular liver carcinoma cell line cells. Human hepatocellular liver carcinoma cell line cells were treated with SCE at 100–300 µg/mL, 20 µM SchA, or 200 µM SchB for 24 hours. Cellular lysates were subject to Western blotting assay. (A) Representative blots of P-gp, MRP2, and OATP1A2. (B–D) Bar graphs to show the expression levels of P-gp, MRP2, and OATP1A2, respectively. Nicotinamide adenine dinucleotide phosphate was used as the internal control. Data are presented as the mean ± standard deviation from at least three independent experiments.
Abbreviations: SCE, Schisandra chinensis extract; Sch, schisandrin; P-gp, P-glycoprotein; MRP2, multidrug resistance-associated protein 2; OATP1A2, organic anion transporting peptide; tBHQ, tert-butylhydroquinone; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Ctrl, control.
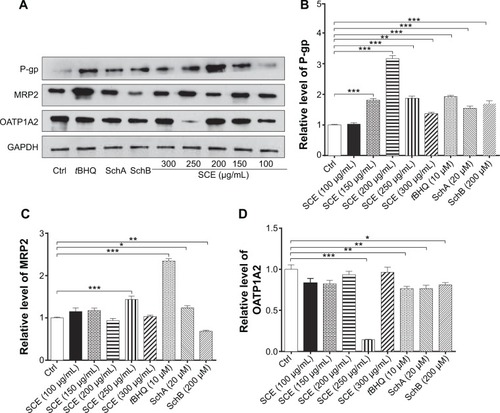
Figure 7 Effect of Schisandra chinensis extract (SCE), schisandrin (Sch) A, and SchB on the intracellular level of glutathione (GSH) and total glutathione S-transferase (GST) content in human hepatocellular liver carcinoma cell line cells. Human hepatocellular liver carcinoma cell line cells were treated with SCE at 100–300 µg/mL, 20 µM SchA, or 200 µM SchB for 24 hours. (A) Intracellular level of GSH. (B) Total GST content. Data are presented as the mean ± standard deviation (n=4).
Abbreviation: Ctrl, control.
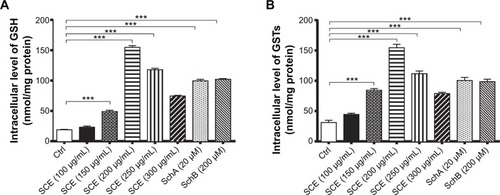
Figure 8 Effect of Schisandra chinensis extract (SCE), schisandrin (Sch) A, and SchB on NAD(P)H:quinone oxidoreductase 1–antioxidant response element–luciferase activity in human hepatocellular liver carcinoma cell line cells. Human hepatocellular liver carcinoma cell line cells were transfected with antioxidant response element– luciferase or control plasmids for 6 hours. Following the transfection, cells were treated with SCE at 50–300 µg/mL, 20 µM SchA, or 200 µM SchB for 24 hours. tert-Butylhydroquinone (tBHQ) at 10 µM was used as a positive control. Luciferase activity was measured using the dual luciferase assay system. Data are presented as the mean ± standard deviation (n=5).
Abbreviation: Ctrl, control.
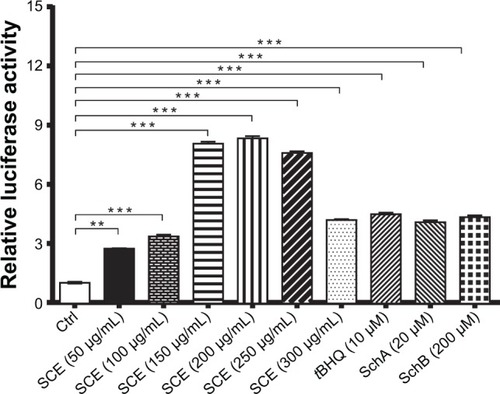
Figure 9 Effect of Nrf2 knockdown by ribonucleic acid interference on the expression of important drug metabolizing enzymes and drug transporters. (A) Transfection efficiency of various Nrf2–small interfering (si) ribonucleic acids in human hepatocellular liver carcinoma cell line cells. (B–E) Bar graphs to show the gene expression levels of Nrf2, NQO1, HO-1, and MRP2, respectively. Data are presented as the mean ± standard deviation (n=4).
Abbreviations: Nrf2, nuclear factor (erythroid-derived 2)-like 2; NQO1, NAD(P)H:quinone oxidoreductase 1; HO-1, heme oxygenase-1; MRP2, multidrug resistance-associated protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; SCE, Schisandra chinensis extract; tBHQ, tert-butylhydroquinone; Sch, schisandrin; Ctrl, control.
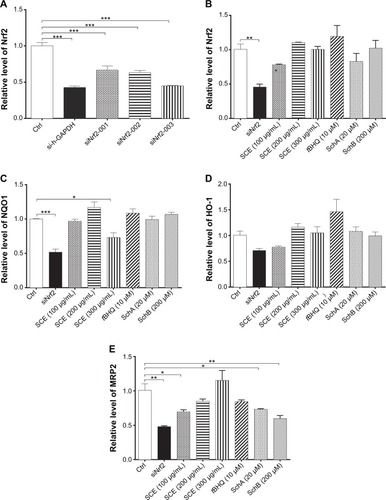
Figure 10 Effect of SCE, SchA, and SchB on the expression of nuclear and total Nrf2 and Keap1. Human hepatocellular liver carcinoma cell line cells were treated with SCE at 100–300 µg/mL, 20 µM SchA, or 200 µM SchB for 24 hours. Whole cellular and nucleic lysates were subject to Western blotting analysis. (A) Representative blots of nuclear and total Nrf2 and Keap1 in human hepatocellular liver carcinoma cell line cells. (B–D) Bar graphs to show the relative expression level of nuclear and total Nrf2 (B and C) and Keap1 (D), respectively. Nicotinamide adenine dinucleotide phosphate was used as the internal control. Data are presented as the mean ± standard deviation (n=3).
Abbreviations: SCE, Schisandra chinensis extract; Sch, schisandrin; Nrf2, nuclear factor (erythroid-derived 2)-like 2; Keap1, Kelch-like ECH-associated protein 1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Ctrl, control.
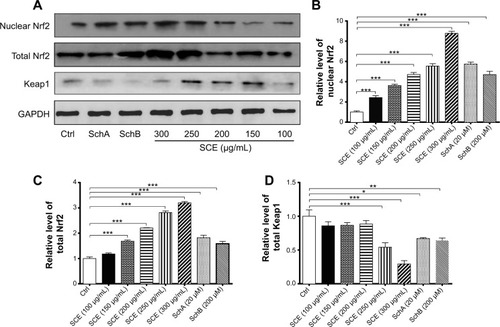
Figure 11 Effect of SCE, SchA, and SchB on Nrf2 protein stability in human hepatocellular liver carcinoma cell line cells.
Abbreviations: SCE, Schisandra chinensis extract; Sch, schisandrin; Nrf2, nuclear factor (erythroid-derived 2)-like 2; CHX, cycloheximide; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; min, minutes.
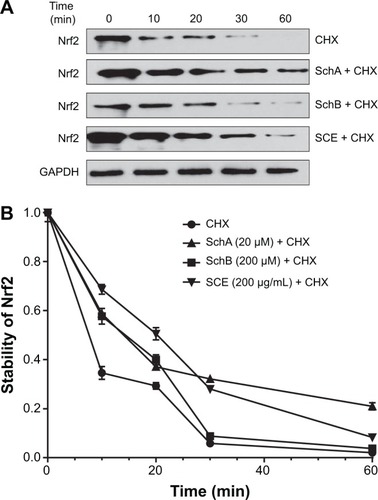
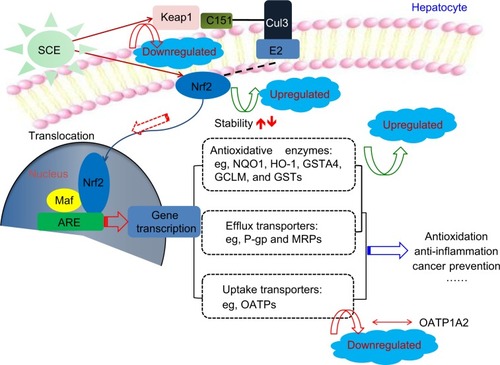
Figure 12 Proposed underlying mechanism for the effect of SCE on drug metabolizing enzymes and drug transporters via Nrf2-mediated signaling pathway. Taken together, our findings have shown that pretreatment of human hepatocellular liver carcinoma cell line cells with SCE increased Nrf2 stability and half-life, mainly via downregulation of Keap1 and inhibition of Nrf2 degradation.