Figures & data
Table 1 Dimensions and weight of fabricated small-size matrix intravaginal rings
Table 2 Dimensions of fabricated PU-93A and PU-60D res-intravaginal ring segments
Figure 1 Drug-free and hydroxychloroquine (HCQ)-loaded matrix and reservoir intravaginal rings (IVRs).
Abbreviation: res-IVRs, reservoir intravaginal rings; XD, cross-sectional diameter; OD, outer diameter; mm, millimeter; PU, polyether urethane.
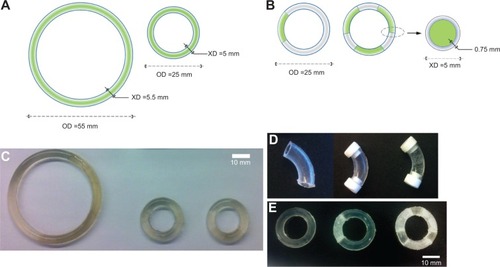
Figure 2 In vitro release of hydroxychloroquine (HCQ) from matrix intravaginal rings (IVRs) and res-IVRs.
Abbreviations: res-IVRs, reservoir intravaginal rings; PVP, polyvinylpyrrolidone; PVA, poly(vinyl alcohol).
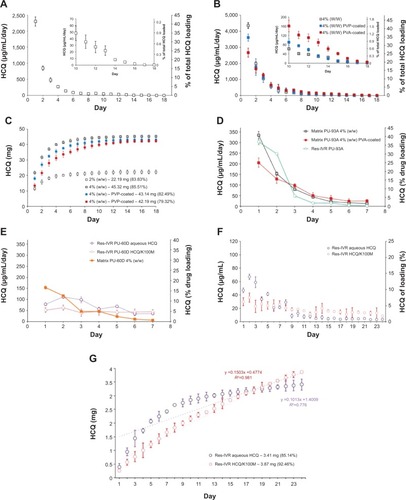
Figure 3 PU-93A matrix intravaginal ring accelerated stability test.
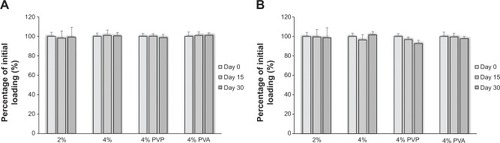
Figure 4 Swelling test of PU-93A matrix intravaginal ring segments.
Abbreviations: PVP, polyvinylpyrrolidone; PVA, poly(vinyl alcohol).
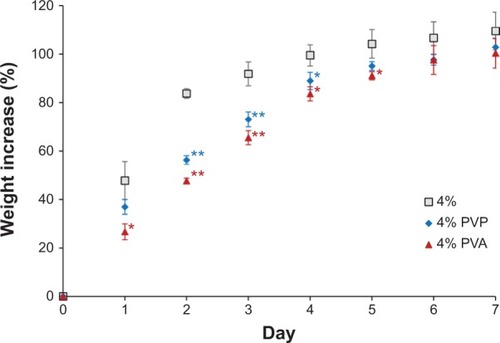
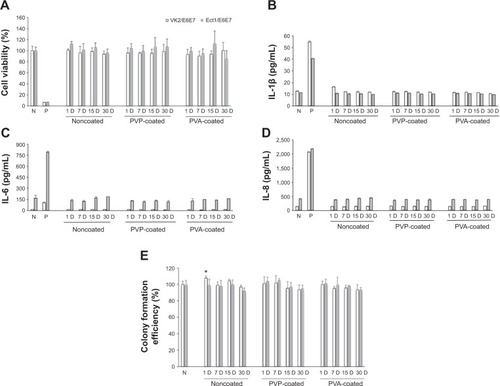
Figure 5 In vitro biocompatibility evaluations of hot-melt intravaginal ring segments.
Abbreviations: N, negative control; P, positive control; PVP, polyvinylpyrrolidone; PVA, poly(vinyl alcohol).